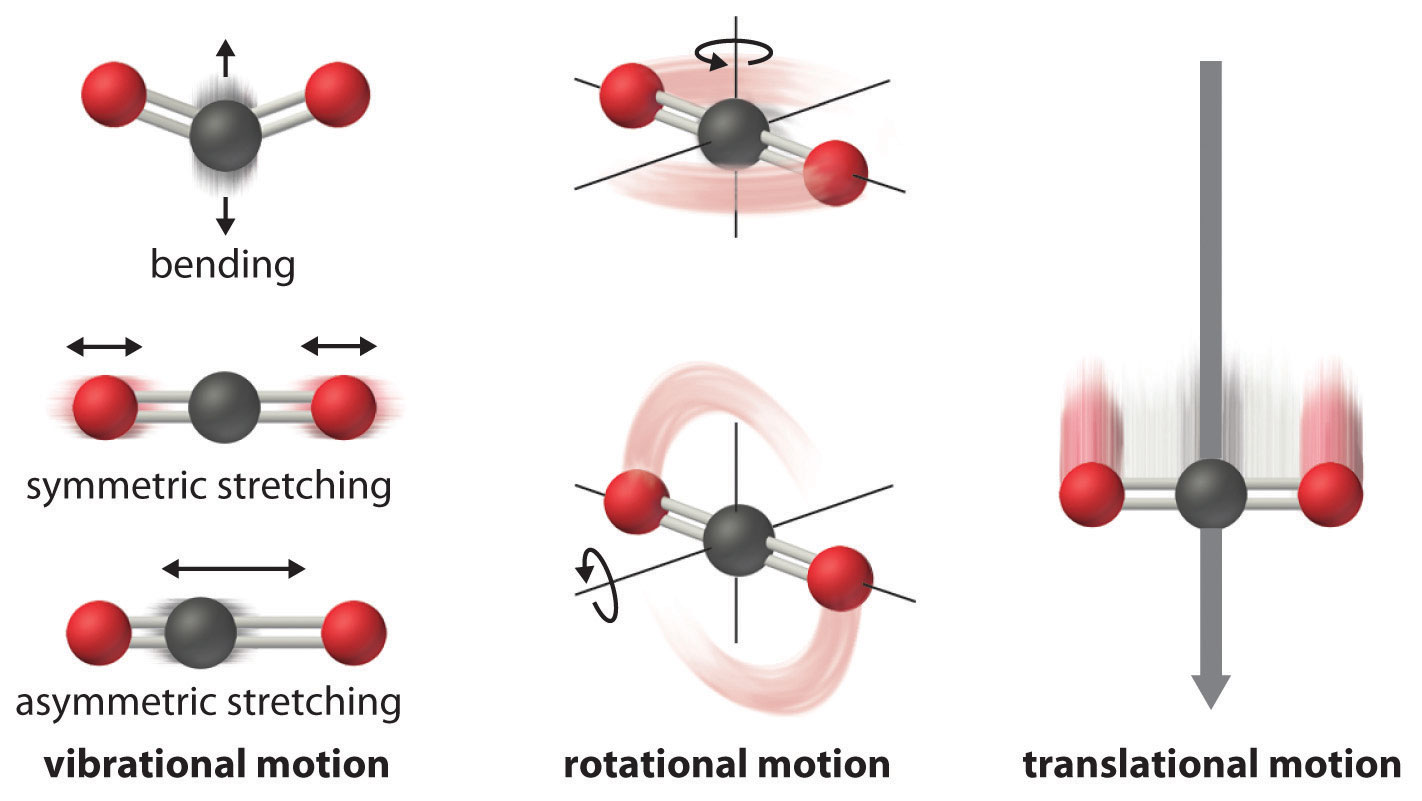
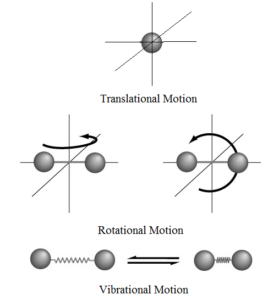
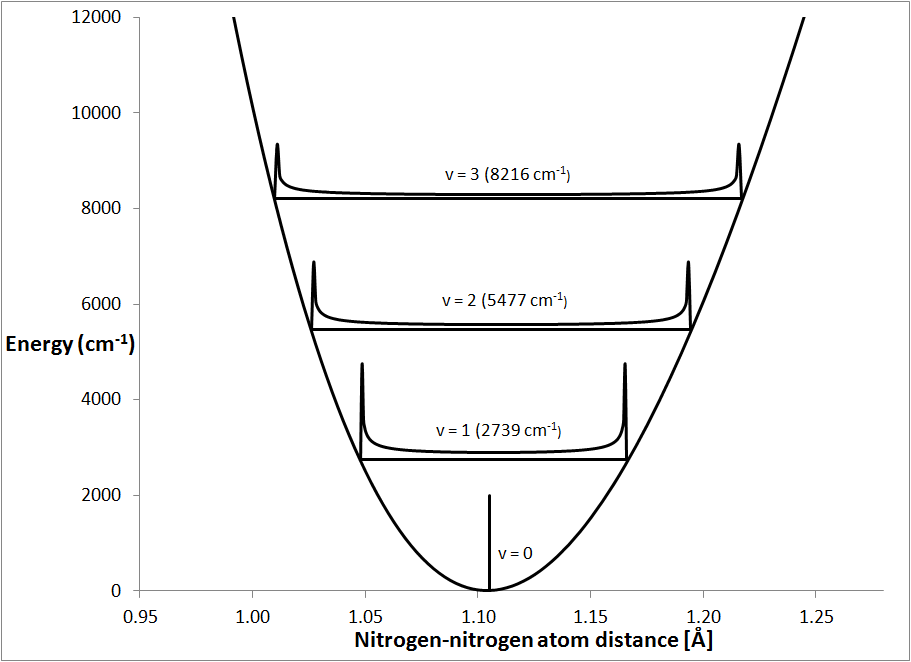
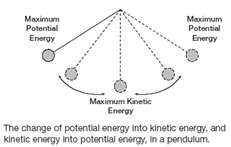
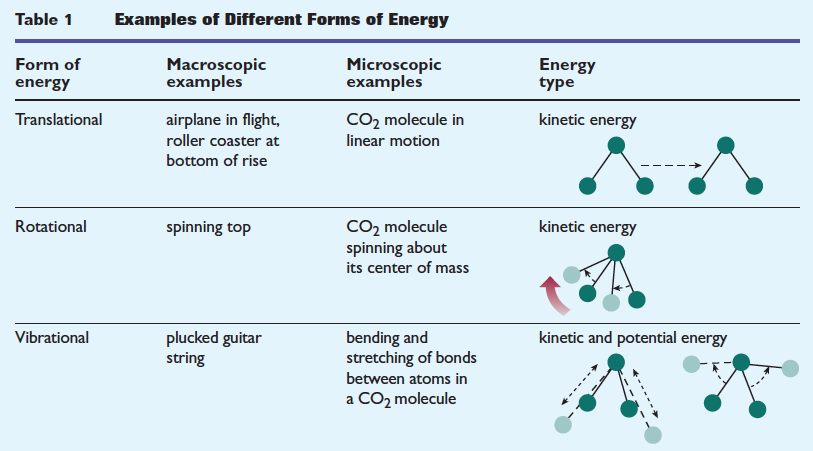
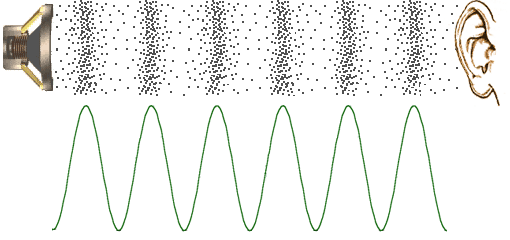
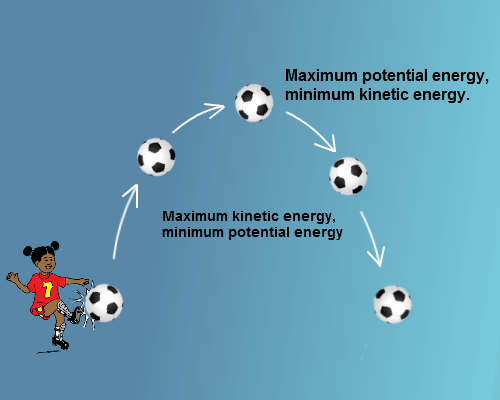
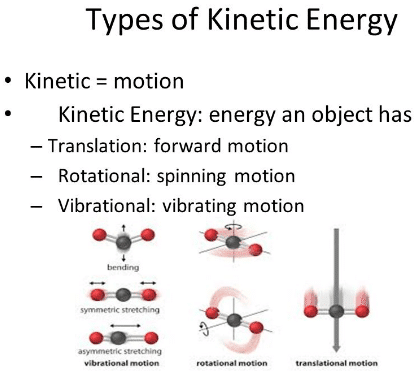
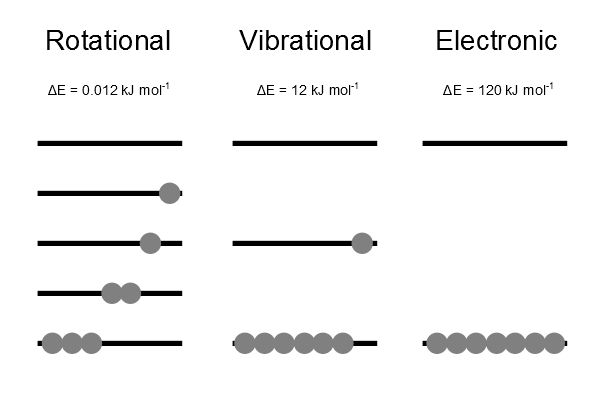
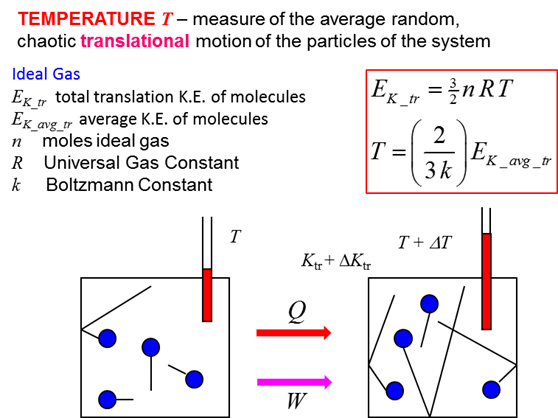
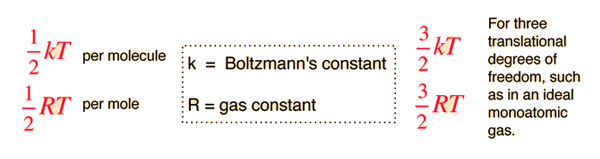Degree of Freedom - Definition, Types, Degree of freedom of mono, di, triatomic gas, Equipartition law of energy with FAQs

CO 2 Vibrational Power Spectrum: Initial kinetic energy on: (a) all... | Download Scientific Diagram

The total vibrational energy of a particle in S.H.M. is E . Its kinetic energy at half the amplitude from mean position will be :

SOLVED: What is the change in energy of the extended system? 4 (Ktot + U) joules What is (Ktot +U)final for the extended system? (Ktot +U )tinal joules What is the final