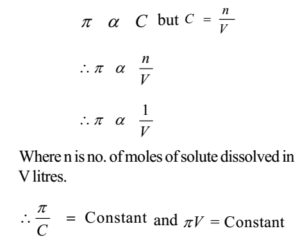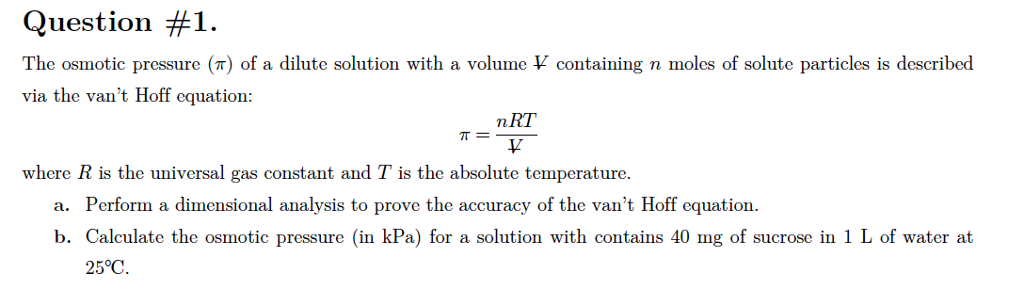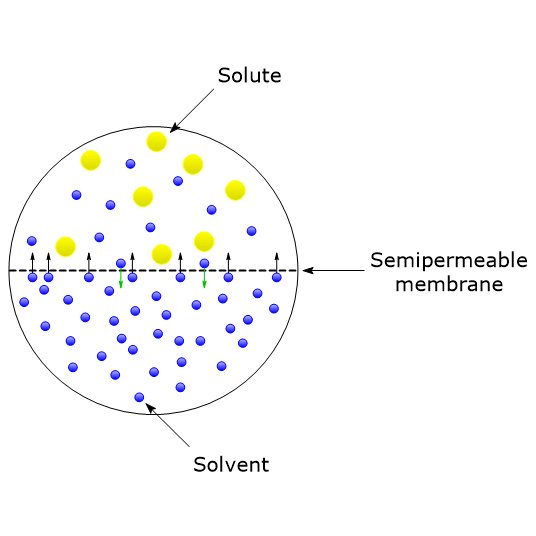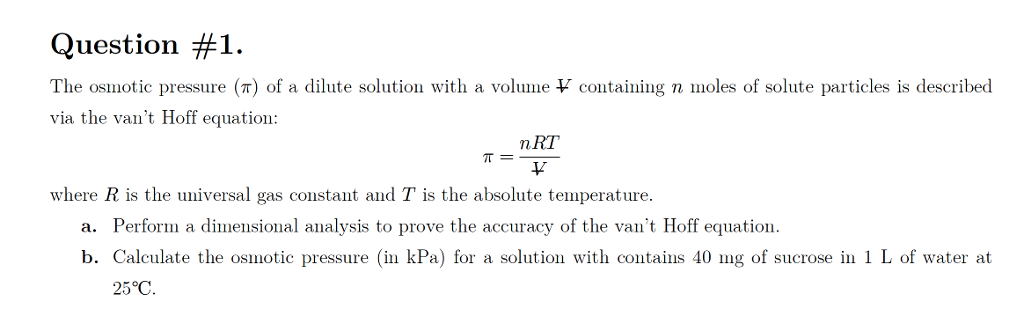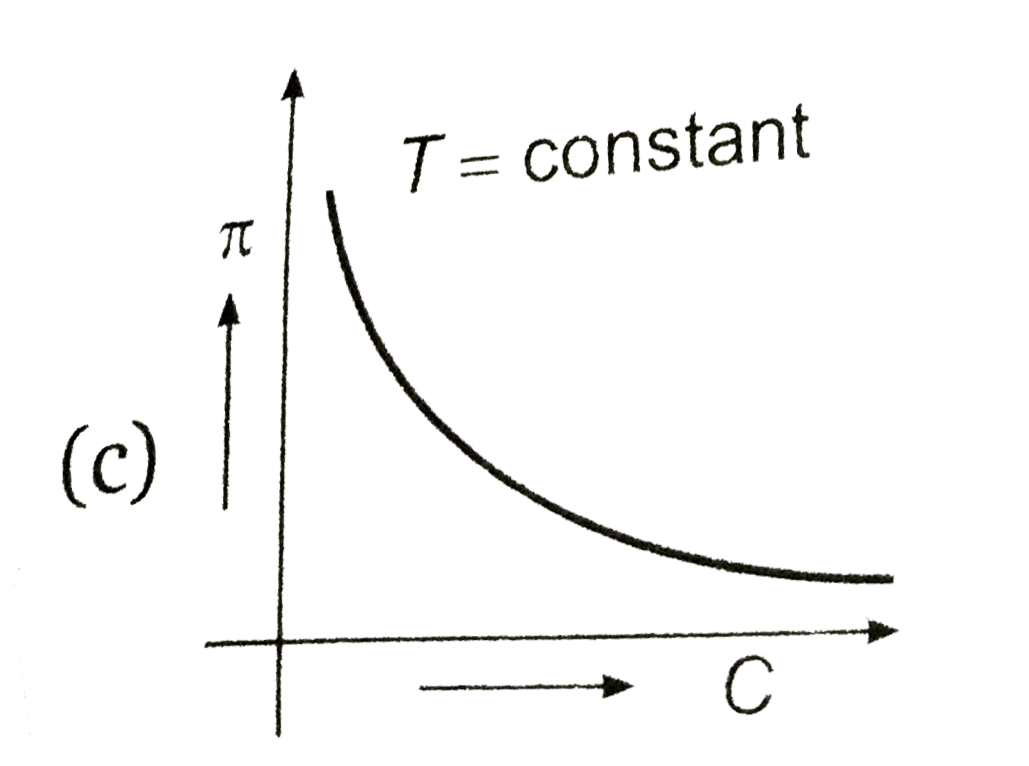
van't Hoff proved that osmotic pressure (pi) is a colligative property. For an ideal solution, osmotic pressure(pi) is helpful to determine that molecular mass of solute using M(B)=(W(B)RT)/(pi.V) Relation can exxpressed by

vant hoff equation for osmotic pressure of solution and expiration of degree of dissolution - Chemistry - - 14038313 | Meritnation.com

Vant Hoff formula - For calculation of Osmotic pressure ... ( Note: Maximum contribution of Plasma osmolarity is by sodi… in 2022 | Osmotic pressure, Gas constant, Pressure
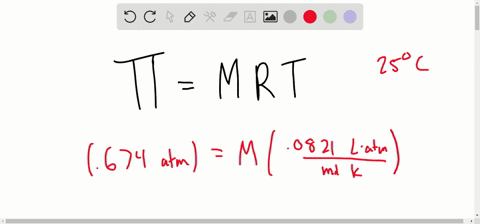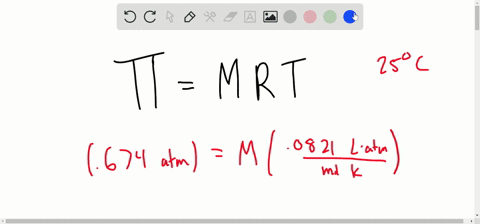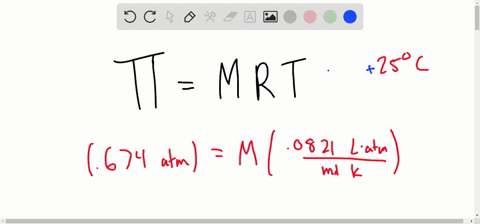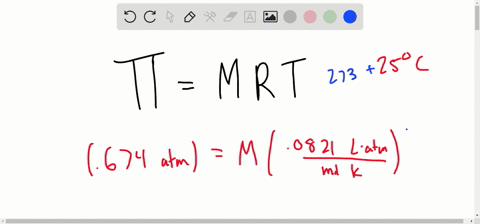
SOLVED:Determine the molarity of each of the following solutions from its osmotic pressure at 25^∘ C . Include the van 't Hoff factor for the solution when the factor is given. a.

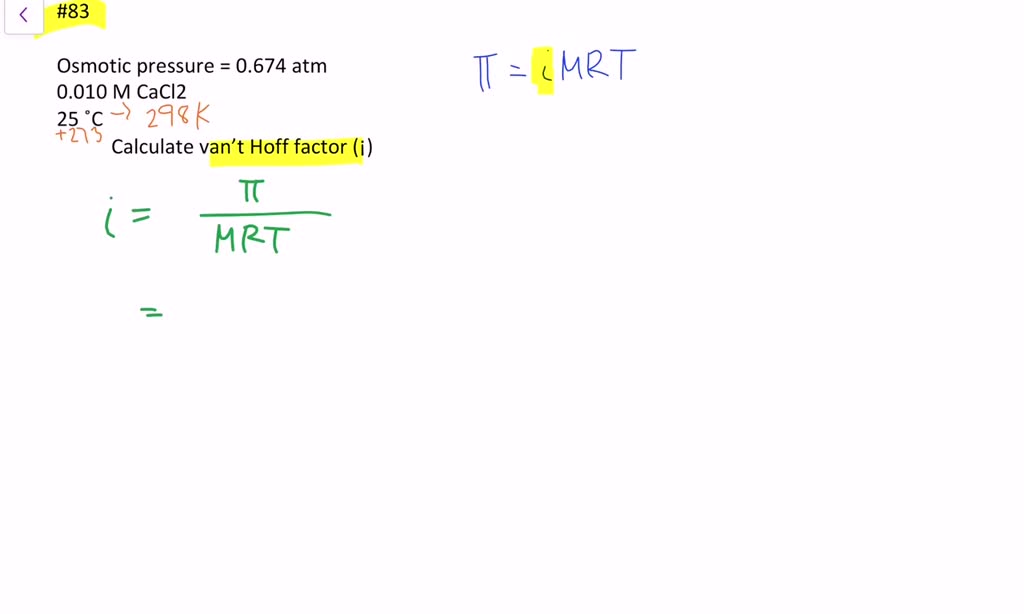
Calculate the amount of CaCl2 (van't Hoff factor i = 2.47 ) dissolved in 2.5 L solution so that its osmotic pressure at 300K is 0.75 atmosphere.Given: Molar mass of CaCl2 is