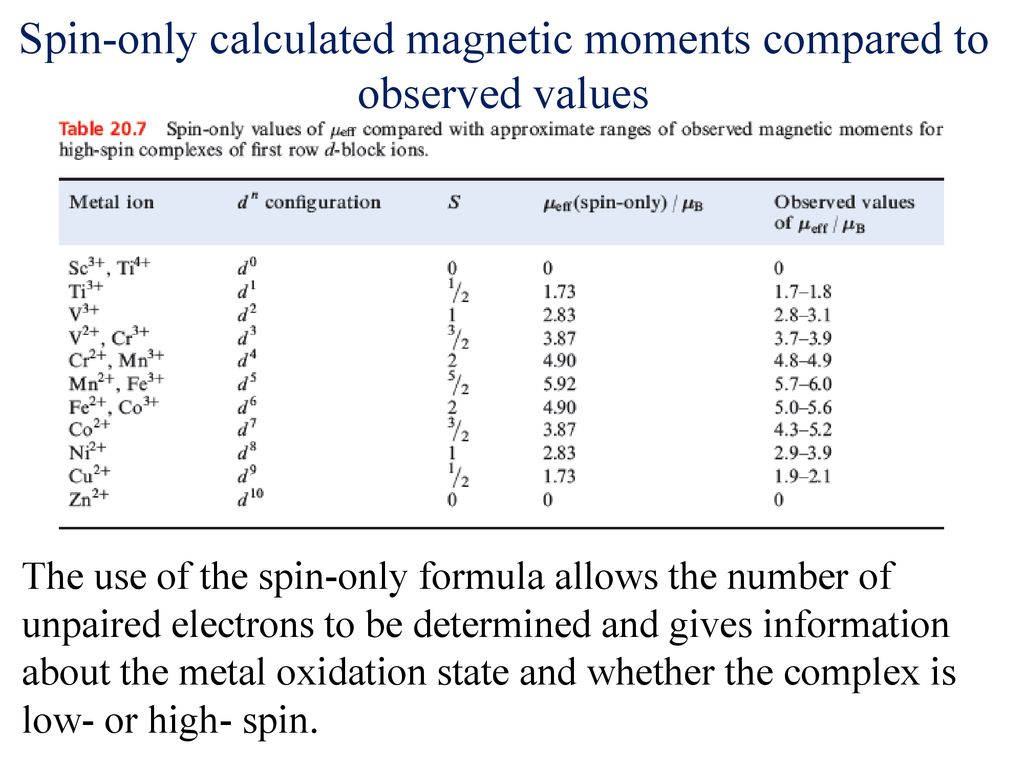
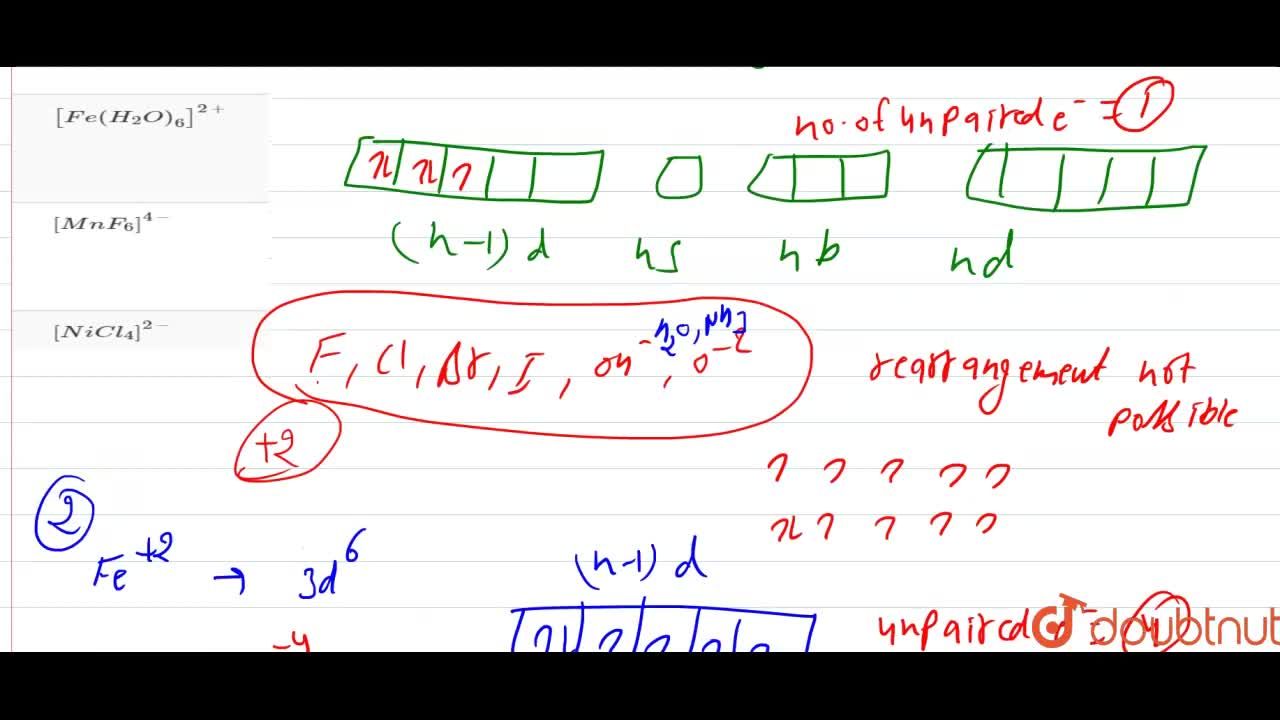
What is the spin-only magnetic moment value (BM) of a divalent metal ion with atomic number 25, in - Sarthaks eConnect | Largest Online Education Community
56.Spin only magnetic moment of Mnx+ ion is root 15B.M.Then what is tge value of X OPTIONS:A)6 B)4 C)2 D)8
Calculate the spin only magnetic moment of La^3+. - Sarthaks eConnect | Largest Online Education Community
![The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` - YouTube The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` - YouTube](https://i.ytimg.com/vi/no_ZGBD689M/maxresdefault.jpg)
The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` - YouTube
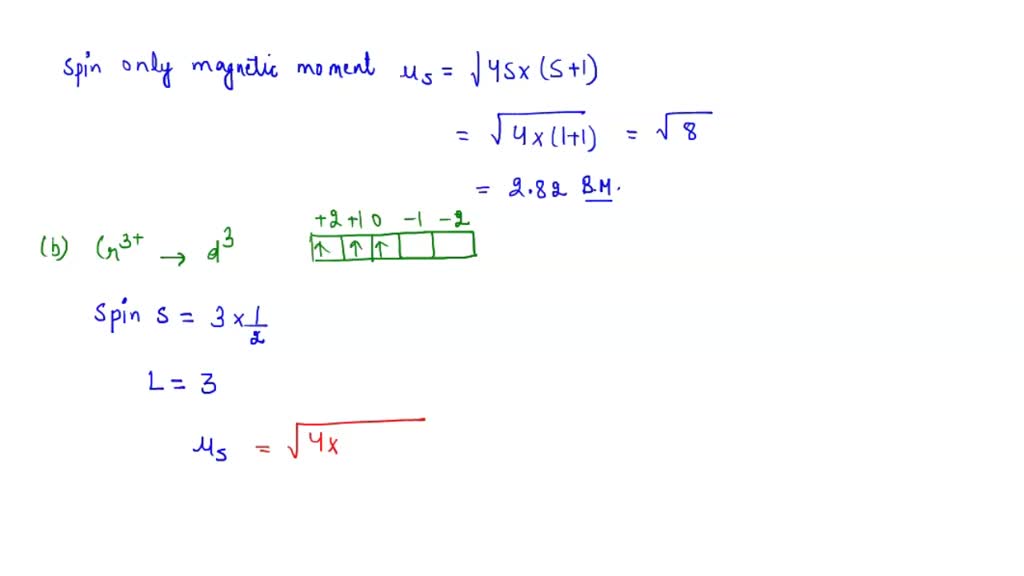
SOLVED: Calculate the magnetic moment of V 3+, Cr3+, Pr3+, Nd3+ according to the following instructions. (a) Consider spin-only magnetic moment in your calculation (b) Consider both spin and orbital moment in
The value of the 'spin only' magnetic moment for one of the following configurations is 2.84 BM. The correct one is - Sarthaks eConnect | Largest Online Education Community
The correct order of the spin-only magnetic moments of the following complexes is : - Sarthaks eConnect | Largest Online Education Community
![The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube](https://i.ytimg.com/vi/KVj56QvOV1I/maxresdefault.jpg)


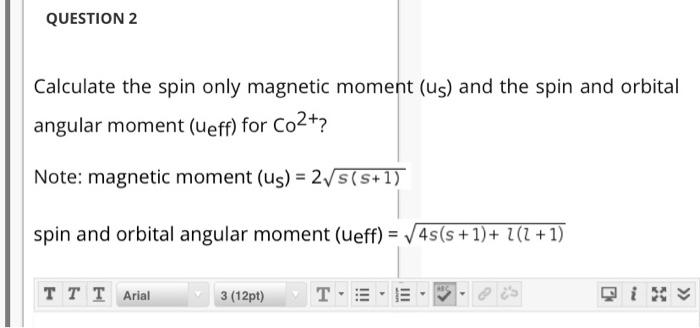


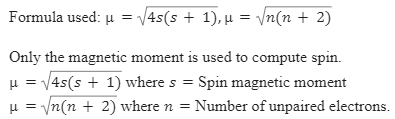


![Spin only magnetic moment of the compound Hg[Co(SCN)4] is : Spin only magnetic moment of the compound Hg[Co(SCN)4] is :](https://d1hhj0t1vdqi7c.cloudfront.net/v1/VzQyTnk4eDZrYms=/sd/)
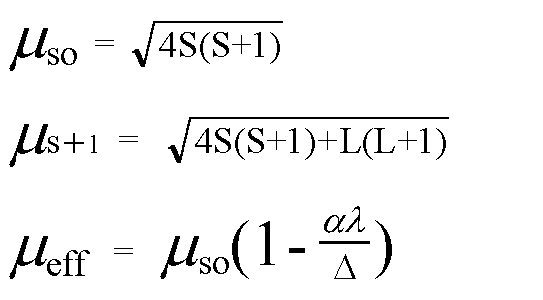

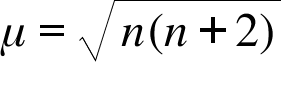
![The spin only magnetic moment of [CrF₆]⁴⁻ (atomic number for Cr is 24) is - NEETLab The spin only magnetic moment of [CrF₆]⁴⁻ (atomic number for Cr is 24) is - NEETLab](https://neetlab.com/wp-content/uploads/2017/11/The-spin-only-magnetic-moment-of-CrF-atomic-number-for-Chemistry-Question-.jpg)