Neoadjuvant treatment of HER 2-positive breast cancer – pathological complete response ( pCR ) as a surrogate of long term outcomes in the context of regulatory guidelines and reimbursement recommendations | Semantic Scholar

Abbreviations: CR, complete response; ORR, objective response rate; PR,... | Download Scientific Diagram
Auditory and sexual preferences for a father's song can co-emerge in female Bengalese finches | PLOS ONE
Physical exercise during neoadjuvant chemotherapy for breast cancer as a mean to increase pathological complete response rates: Trial protocol of the randomized Neo-ACT trial | PLOS ONE
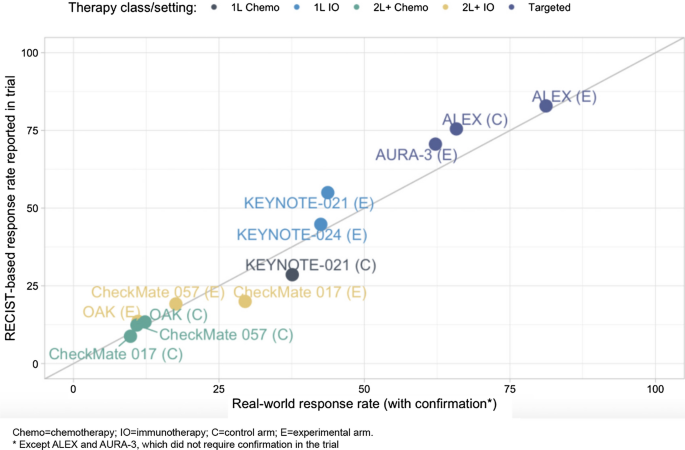
Characterization of a Real-World Response Variable and Comparison with RECIST-Based Response Rates from Clinical Trials in Advanced NSCLC | SpringerLink
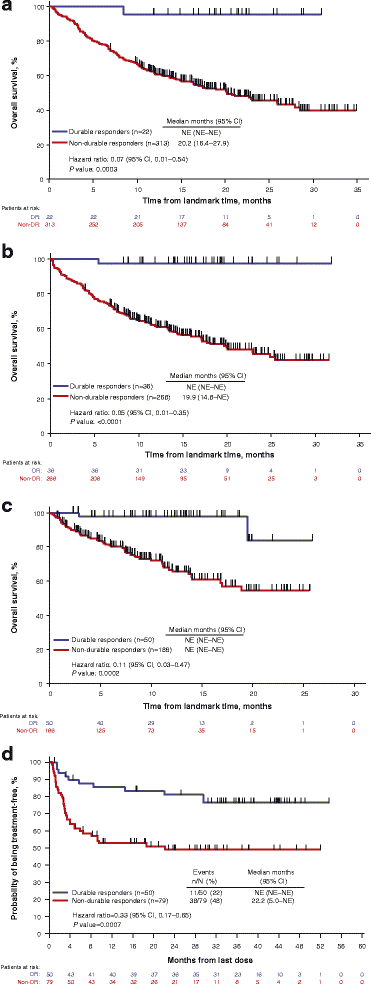
Durable response rate as an endpoint in cancer immunotherapy: insights from oncolytic virus clinical trials | Journal for ImmunoTherapy of Cancer | Full Text
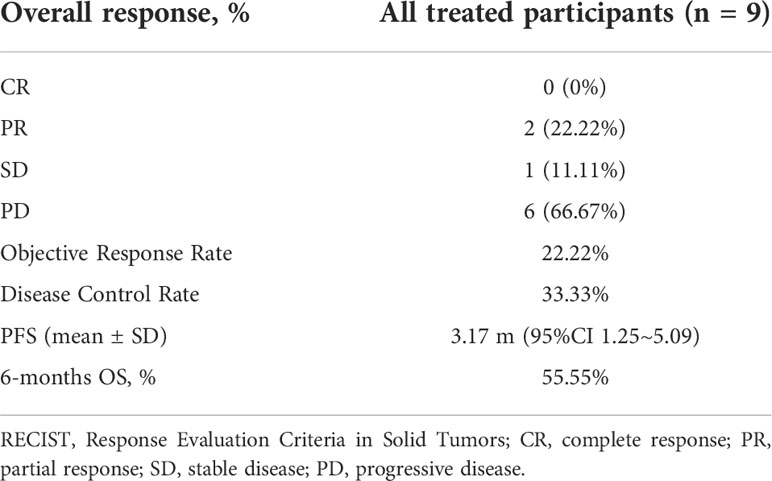
Frontiers | Investigation of the efficacy and safety of cryoablation and intra-arterial PD-1 inhibitor in patients with advanced disease not responding to checkpoint inhibitors: An exploratory study
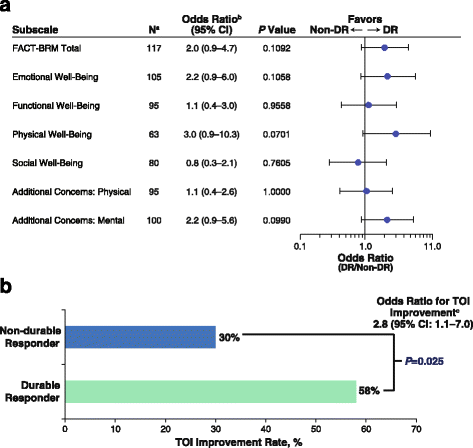
Durable response rate as an endpoint in cancer immunotherapy: insights from oncolytic virus clinical trials | Journal for ImmunoTherapy of Cancer | Full Text

Overall response rate (CR, CRi, PR, and HI) by baseline characteristics | Download Scientific Diagram

Relationship Between Progression‐Free Survival, Objective Response Rate, and Overall Survival in Clinical Trials of PD‐1/PD‐L1 Immune Checkpoint Blockade: A Meta‐Analysis - Ye - 2020 - Clinical Pharmacology & Therapeutics - Wiley Online

Response rates Overall response rate and depth of response according to... | Download Scientific Diagram