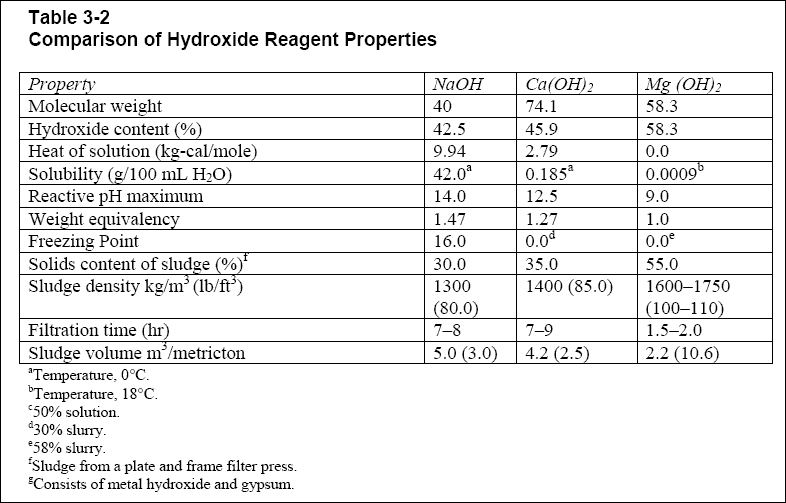
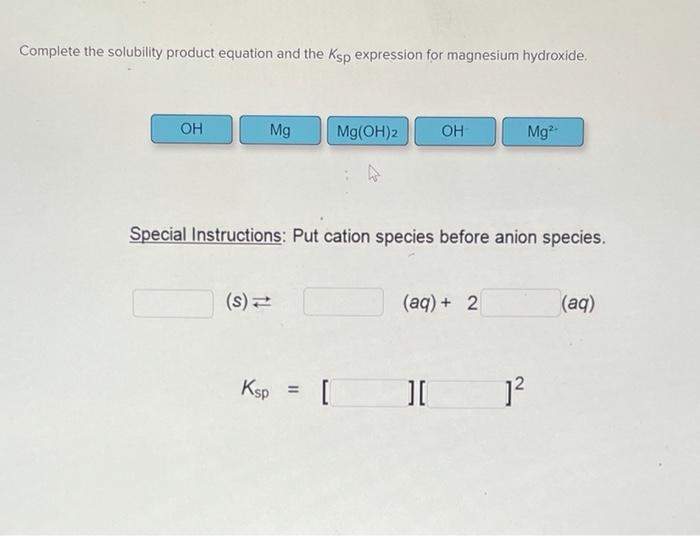
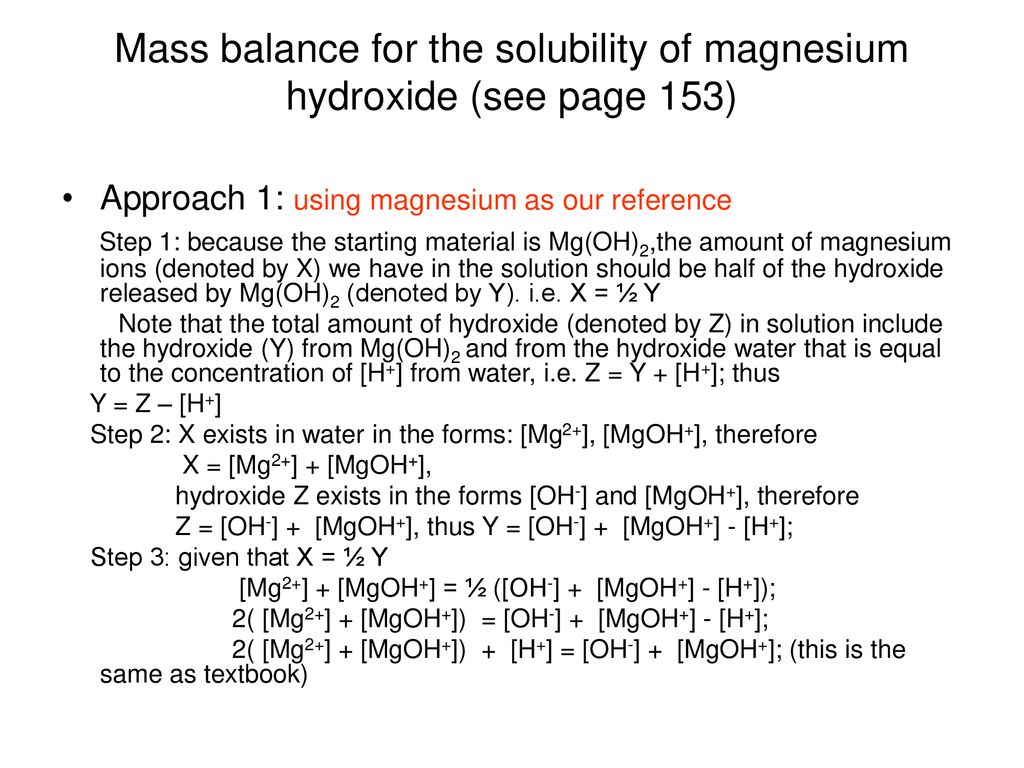
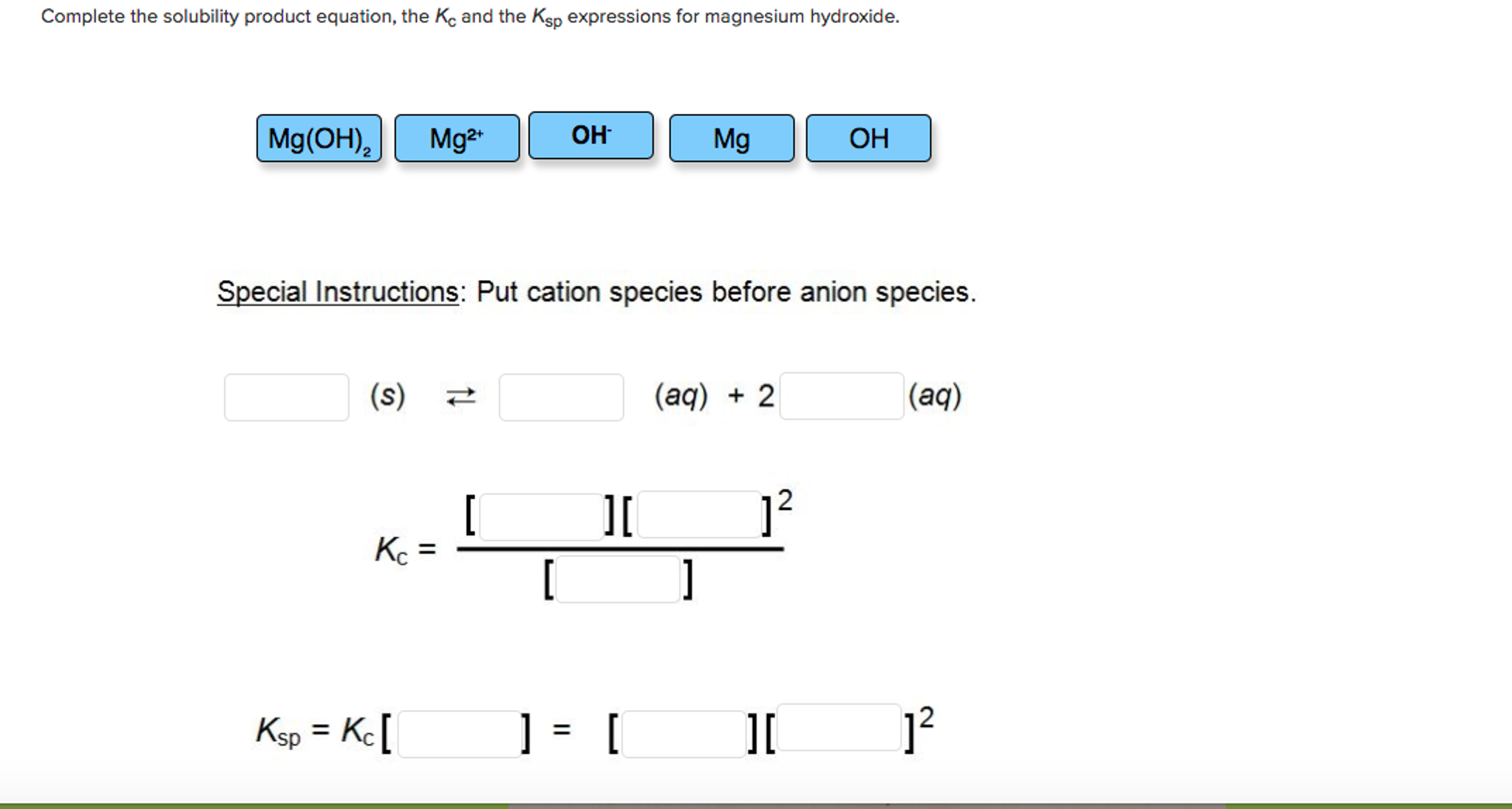
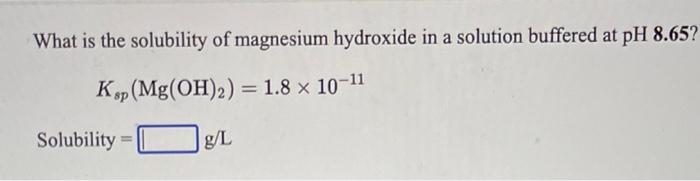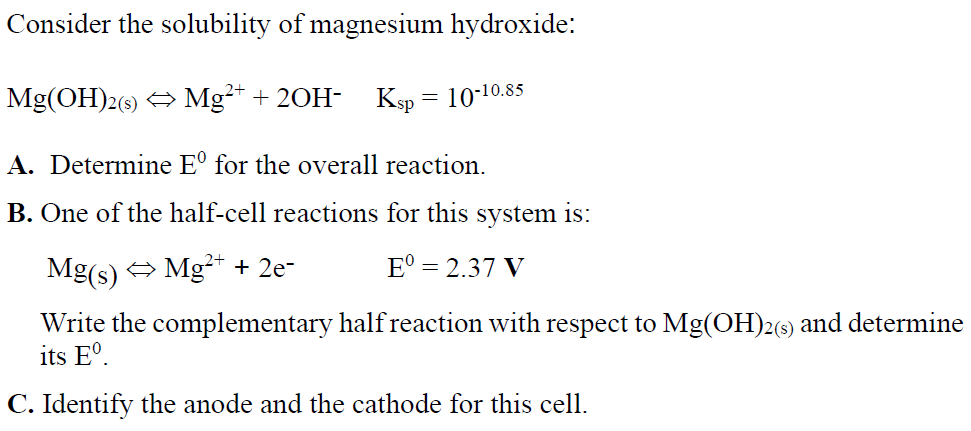The solubility product of magnesium hydroxide at 25 ^(@)C is .1.8 xx 10 ^(-11). Calculate the solubility of magnesium hydroxide.
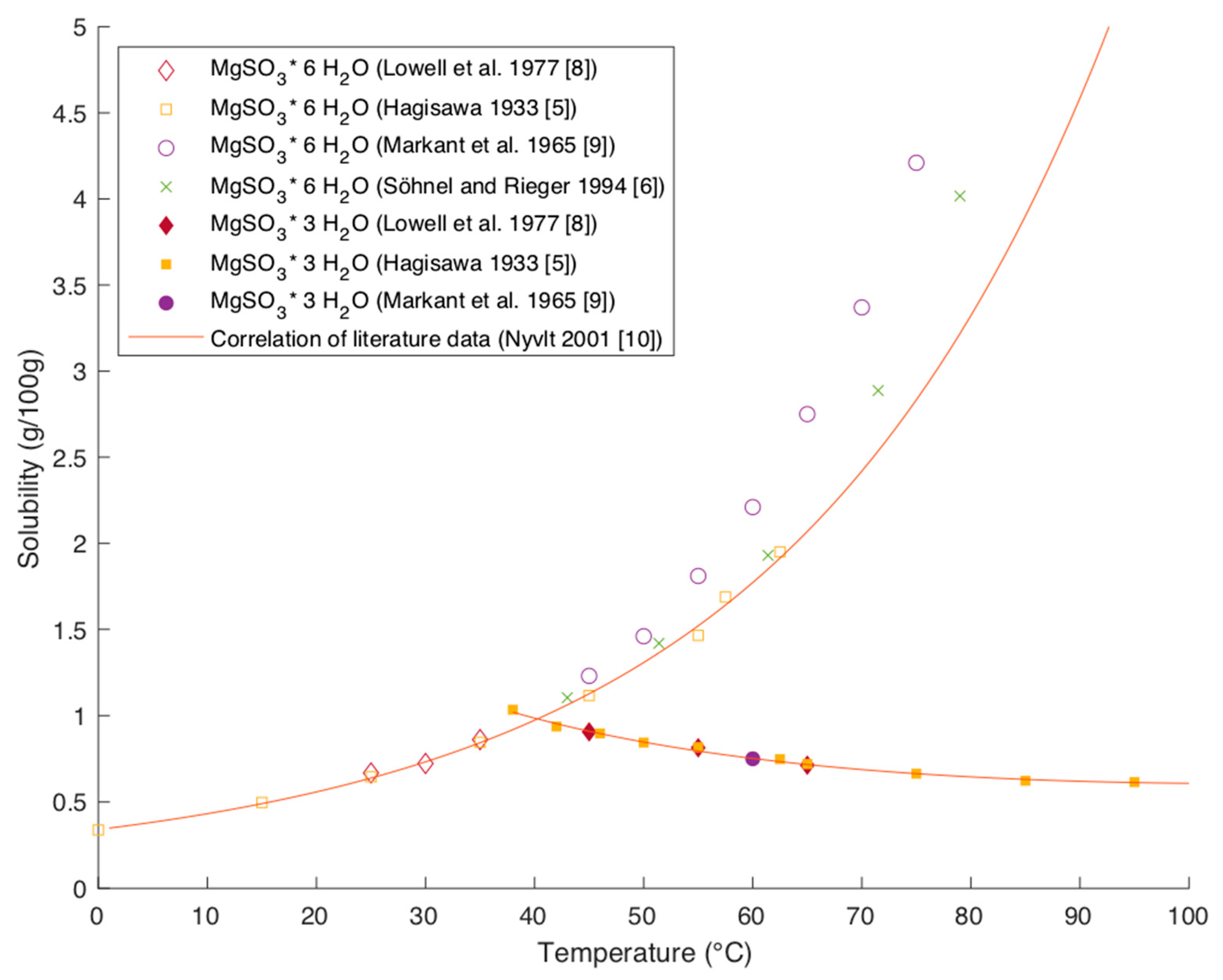
Processes | Free Full-Text | Solubility Data of Potential Salts in the MgO-CaO-SO2-H2O-O2 System for Process Modeling
![SOLVED: Calculate the solubility constant for dissolving Magnesium hydroxide Mg(OH) in water if [Mgl is 0.014 Problem 3: Calculate the solubility constant for dissolving Calcium hydroxide Ca(OH)z in water if [Ca ] SOLVED: Calculate the solubility constant for dissolving Magnesium hydroxide Mg(OH) in water if [Mgl is 0.014 Problem 3: Calculate the solubility constant for dissolving Calcium hydroxide Ca(OH)z in water if [Ca ]](https://cdn.numerade.com/ask_images/e1aaca9121da4955a58de8b427babda4.jpg)
SOLVED: Calculate the solubility constant for dissolving Magnesium hydroxide Mg(OH) in water if [Mgl is 0.014 Problem 3: Calculate the solubility constant for dissolving Calcium hydroxide Ca(OH)z in water if [Ca ]
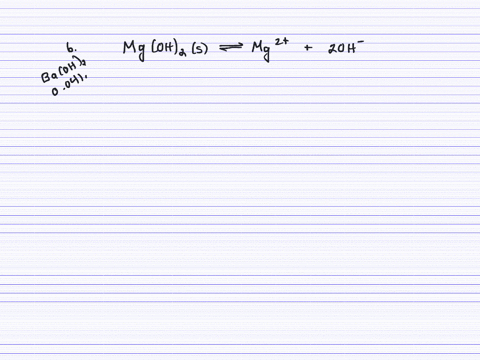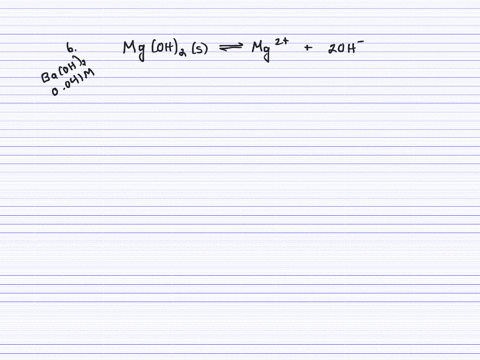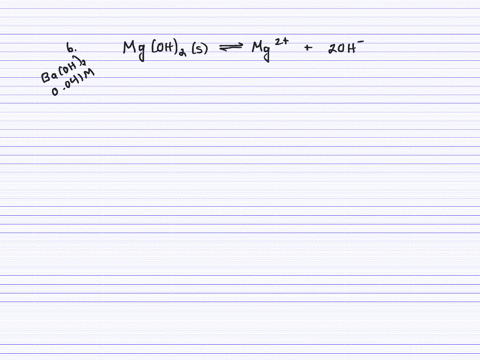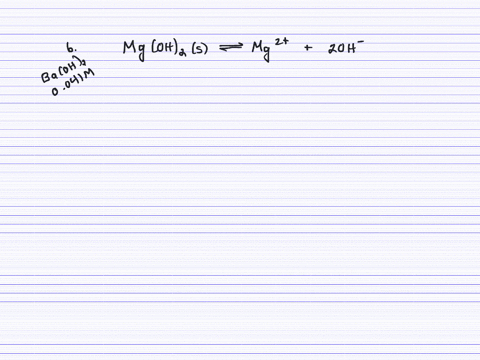
SOLVED:Calculate the solubility (in grams per liter) of magnesium hydroxide in the following. (a) pure water (b) 0.041 M Ba(OH)2 (c) 0.0050 M MgCl2
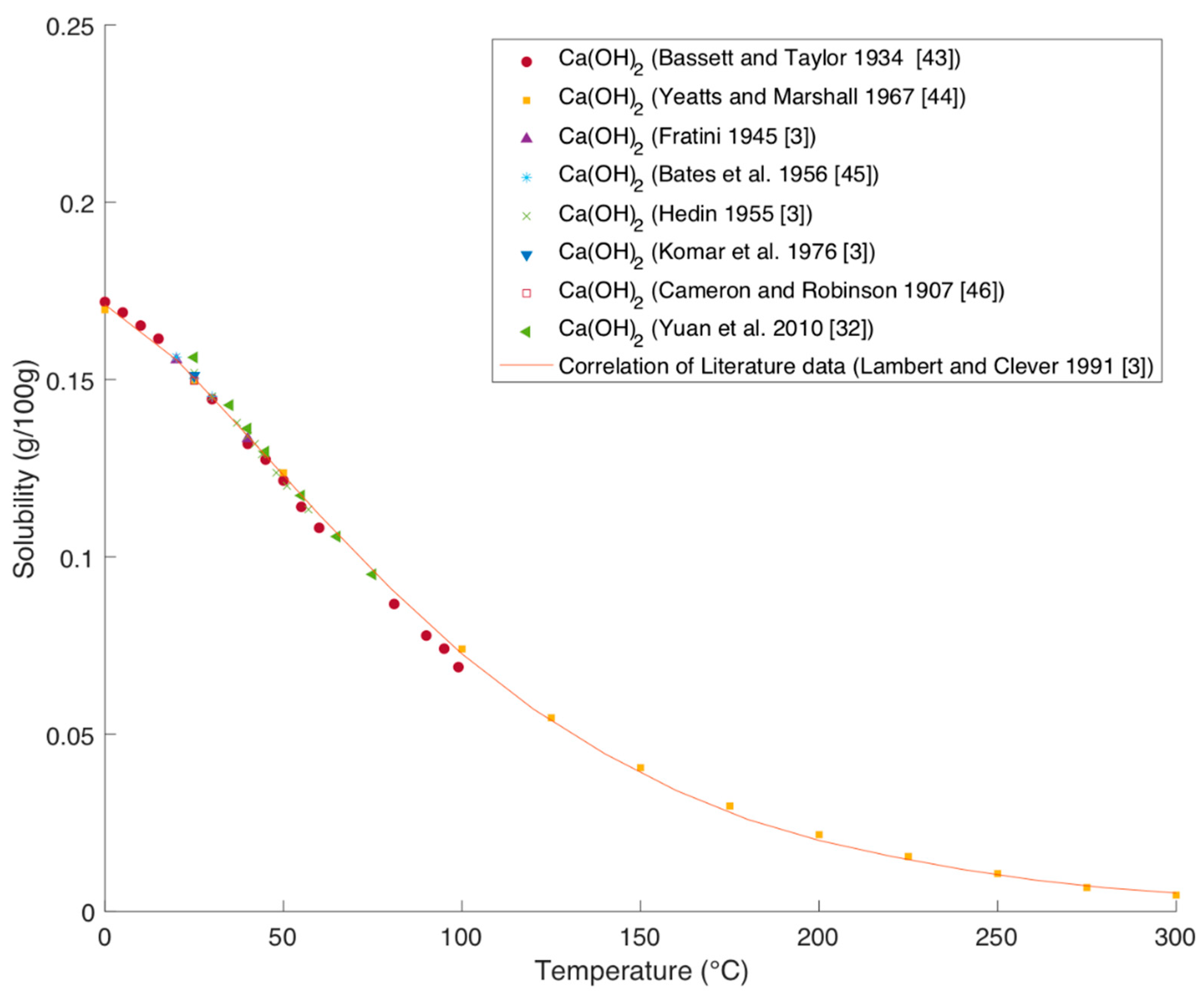
Processes | Free Full-Text | Solubility Data of Potential Salts in the MgO-CaO-SO2-H2O-O2 System for Process Modeling
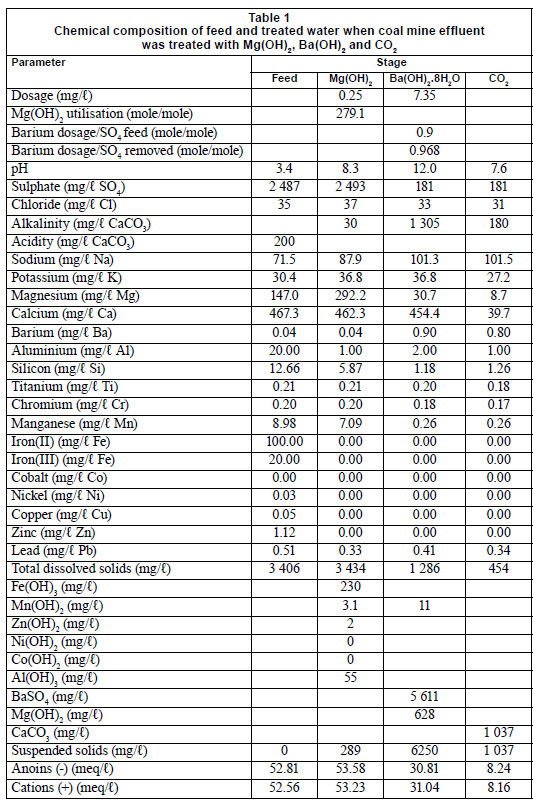
Application of magnesium hydroxide and barium hydroxide for the removal of metals and sulphate from mine water


![Solved [15 points] Consider the solubility of magnesium | Chegg.com Solved [15 points] Consider the solubility of magnesium | Chegg.com](https://media.cheggcdn.com/media%2Fab0%2Fab0a867e-425d-498d-8eeb-8f1ffaa9b69d%2FphpGrMmFr.png)