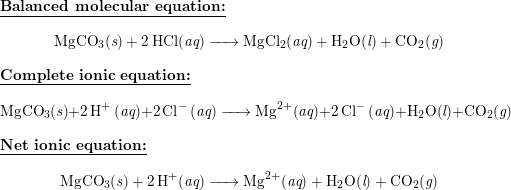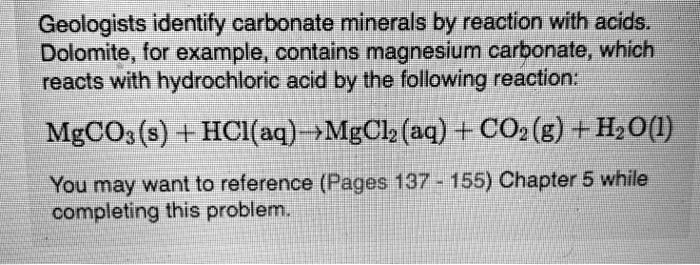
SOLVED: Geologists identify carbonate minerals by reaction with acids Dolomite , for example , contains magnesium carbonate , which reacts with hydrochloric acid by Ihe following reaction: MgCOs (s) + HCl(aq) ,MgClz (
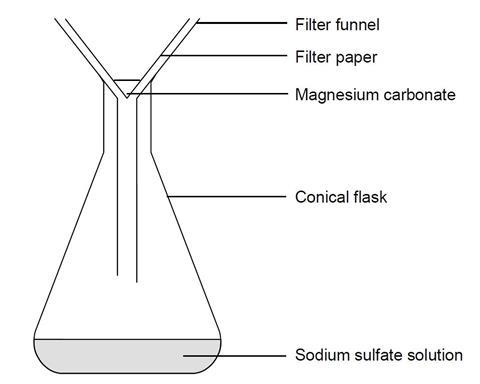
Making magnesium carbonate: the formation of an insoluble salt in water | Experiment | RSC Education
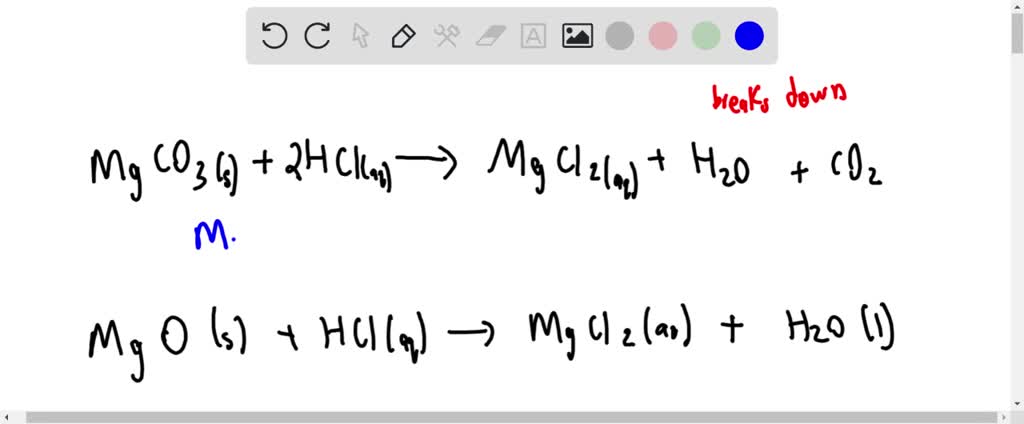
SOLVED:Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that react with acidic solutions. (a) Write a balanced molecular equation and a net ionic equation for the reaction that occurs
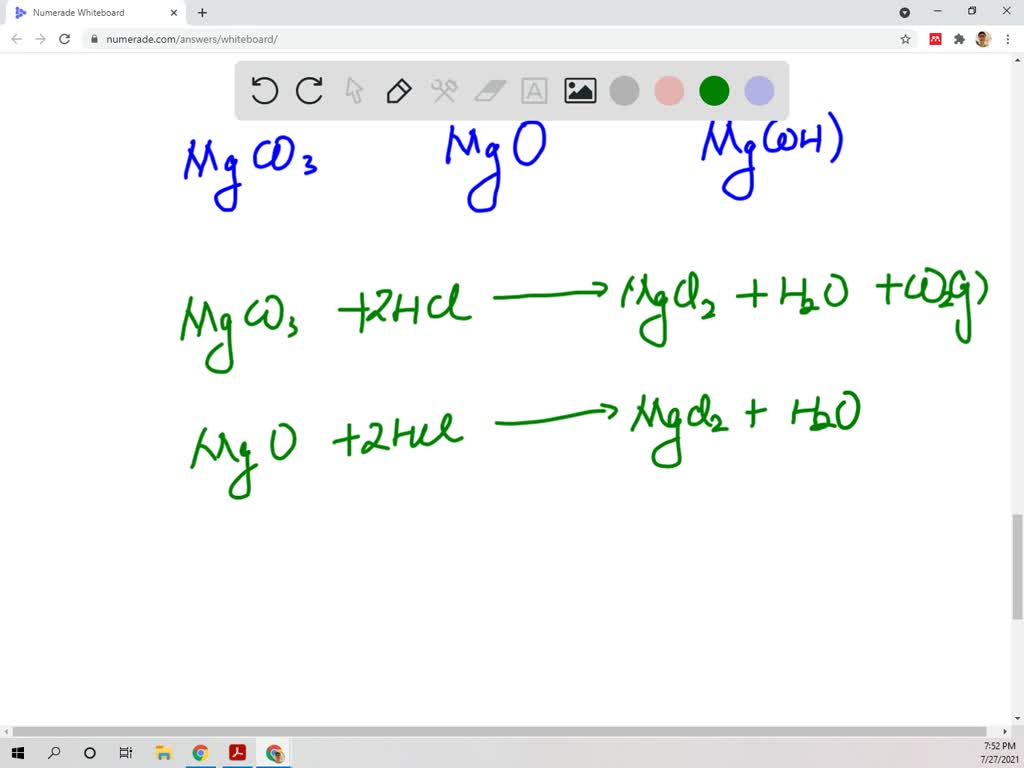
SOLVED: Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that react with acidic solutions. (a) Write a balanced molecular equation and a netionic equation for the reaction that occurs

What is magnesium carbonate?. Magnesium carbonate is an inorganic… | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
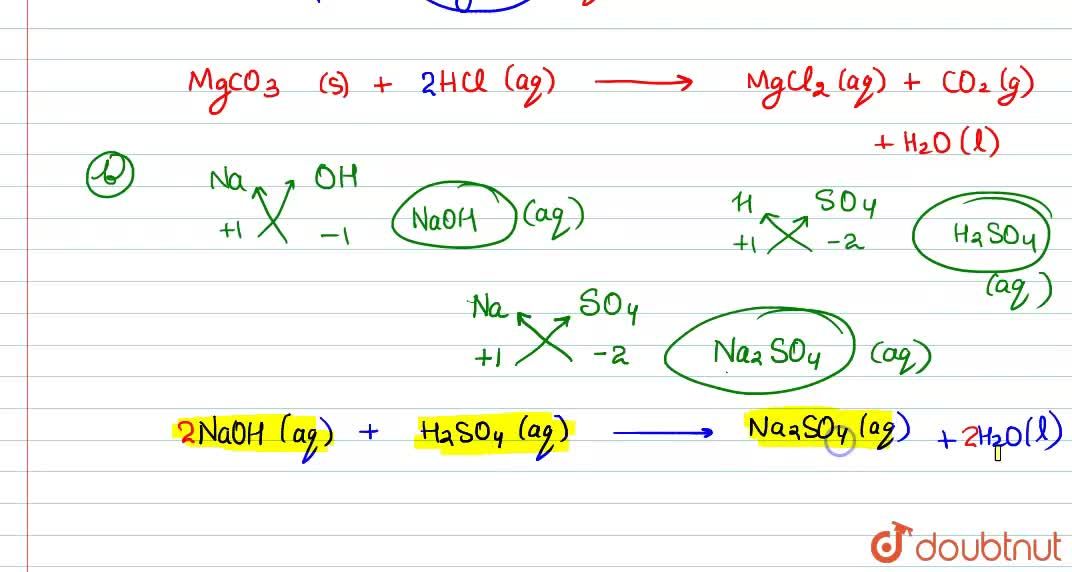
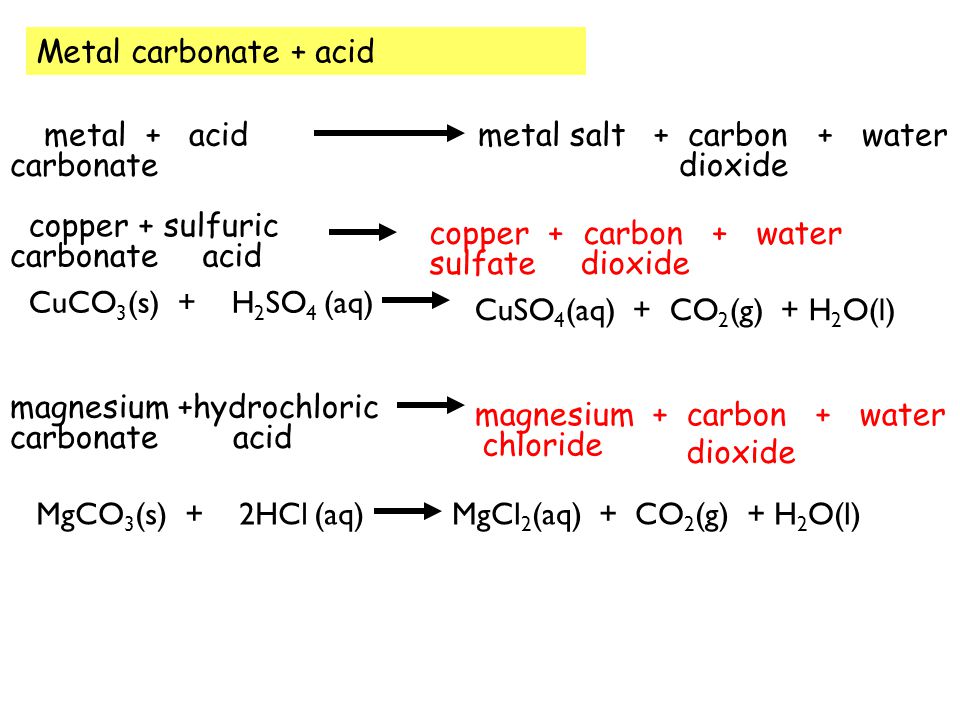
Write the balanced equations for the following reactions, and add the state symbols : (a) Magnesium carbonate reacts with hydrocloric acid to produce magnesium chloride, carbon dioxide and water. (b) Sodium hydroxide

SOLVED:The mineral dolomite contains magnesium carbonate. This reacts with hydrochloric acid. MgCO3(s)+2 HCl(aq) →CO2(g)+MgCl2(aq)+H2 O(ℓ) (a) Write the net ionic equation for this reaction and identify the spectator ions. (b) What type

Magnesium carbonate reacts with hydrochloric acid to form magnesium chloride, carbon dioxide and water Translate and balance the equation - Science - Chemical Reactions and Equations - 12554199 | Meritnation.com