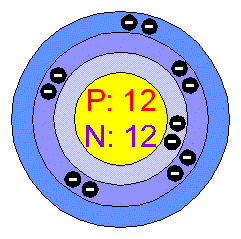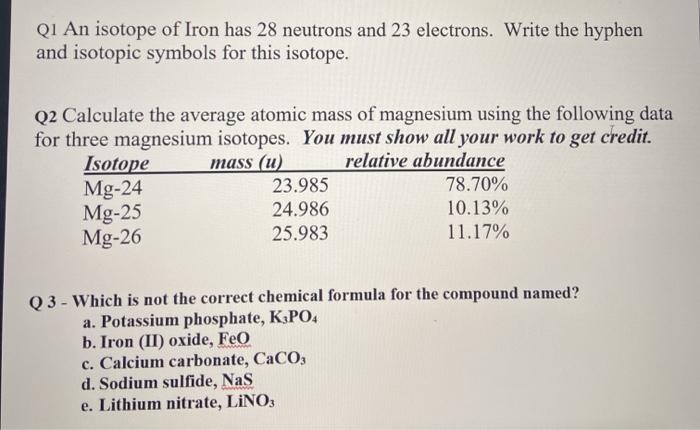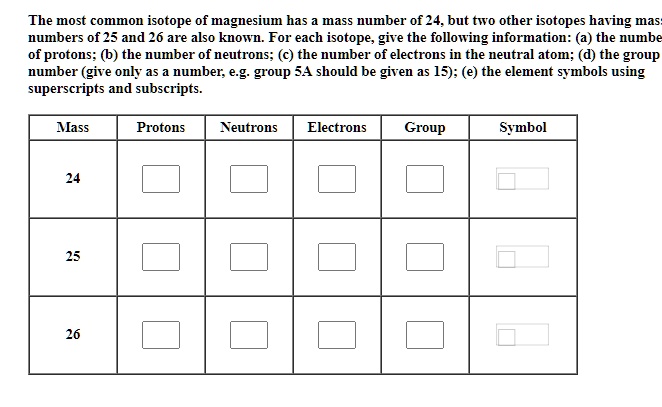
SOLVED: The most common isotope of magnesium has mass number of 24. but two other isotopes having mas numbers of 25 and 26 are also known For each isotope; give the following

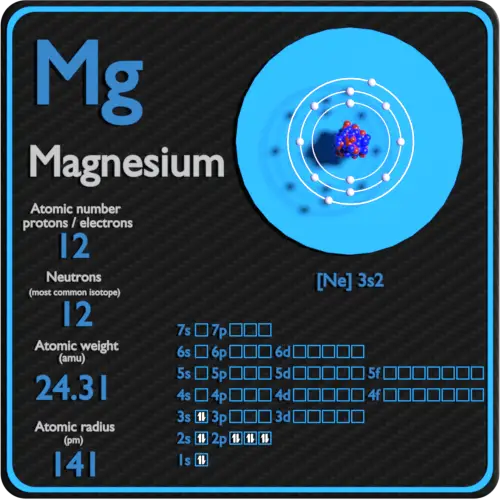
Isotopes. Isotopes of Magnesium Atomic symbol Mg Mg Mg Number of protons Number of electrons Mass number Number of neutrons ppt download

What is the Difference Between Magnesium Atom and Magnesium Ion | Compare the Difference Between Similar Terms
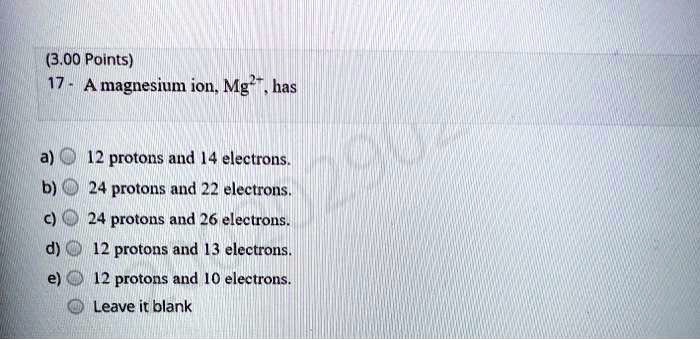
SOLVED: (3.00 Points) A magnesium ion. Mg has 12 protons and [4 electrons 24 protons and 22 electrons. 24 protons and 26 electrons. 12 protons and [3 electrons. 12 protons and [0 electrons. Leave it blank
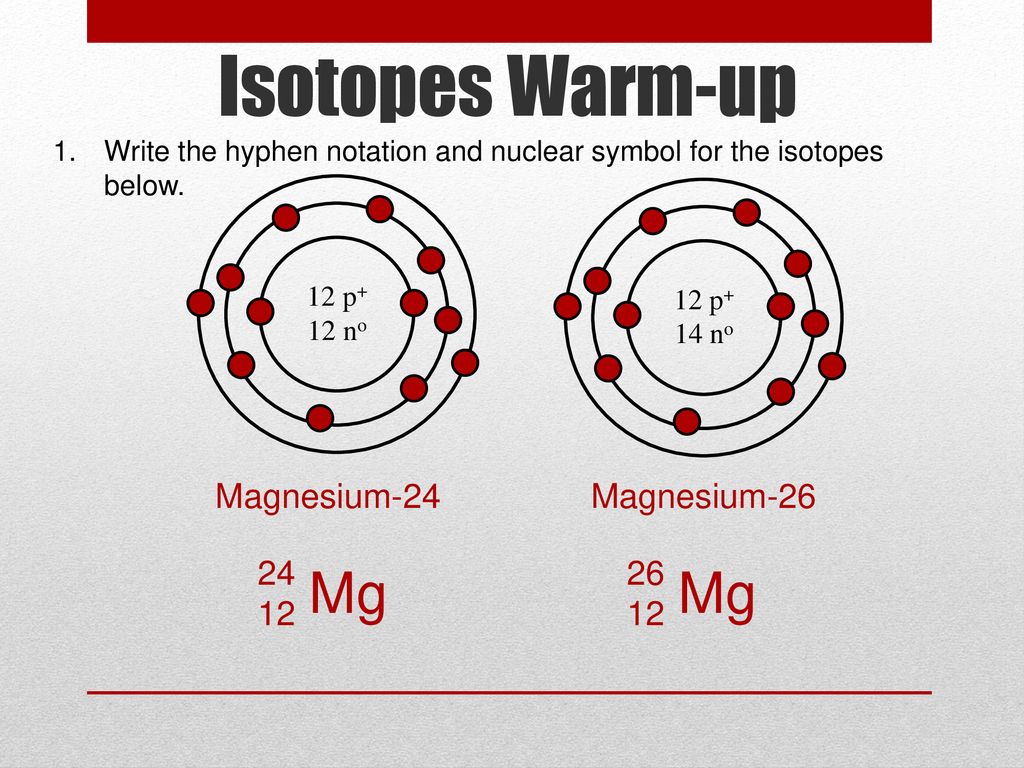
Isotopes Warm-up Write the hyphen notation and nuclear symbol for the isotopes below. 12 p+ 12 no 12 p+ 14 no 2. A atom has 26 protons and 30 neutrons. - ppt download
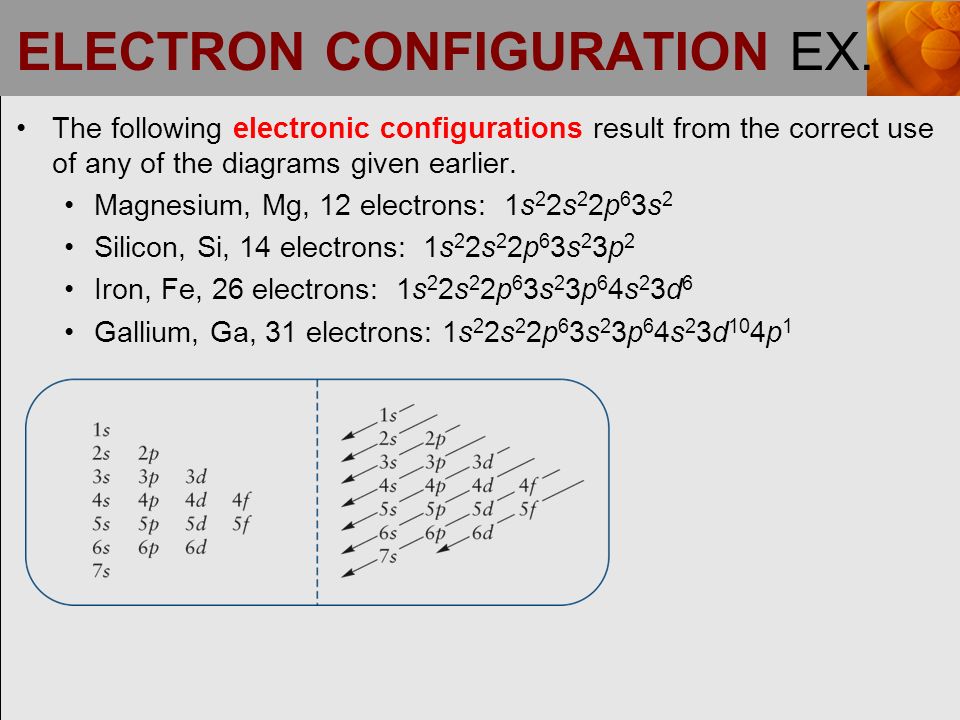
ELECTRON CONFIGURATION EX. The following electronic configurations result from the correct use of any of the diagrams given earlier. Magnesium, Mg, ppt download
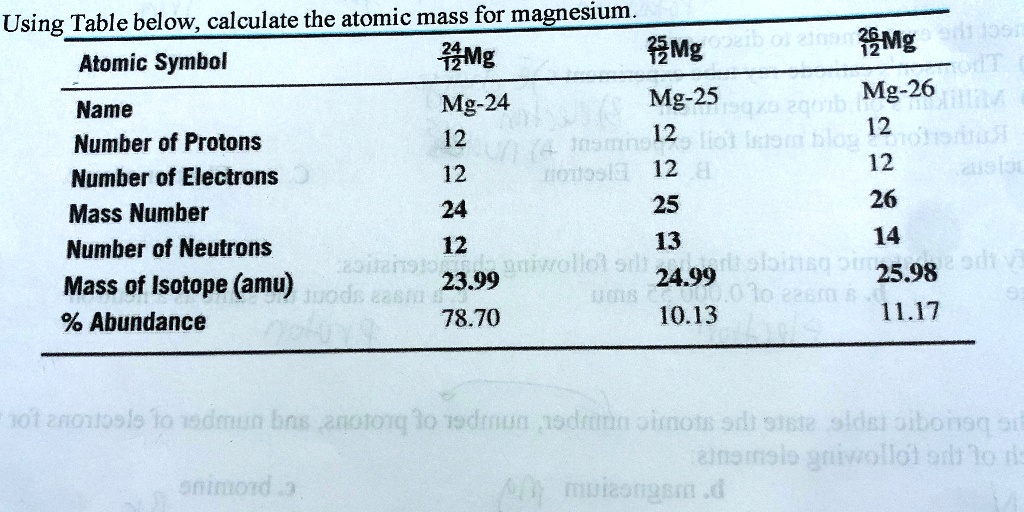
SOLVED: Using Table below,calculate the atomic mass for magnesium iMg 2Mg Atomic Symbol #Mg Mg-24 Mg-25 equtb Mg-26 Name 4 ^ Number of Protons 12 Jetttig12 io Unotb 12 "a 12 Wyoon






:max_bytes(150000):strip_icc()/GettyImages-1135707671-640473b29d534e15a24491c0d6b2789e.jpg)