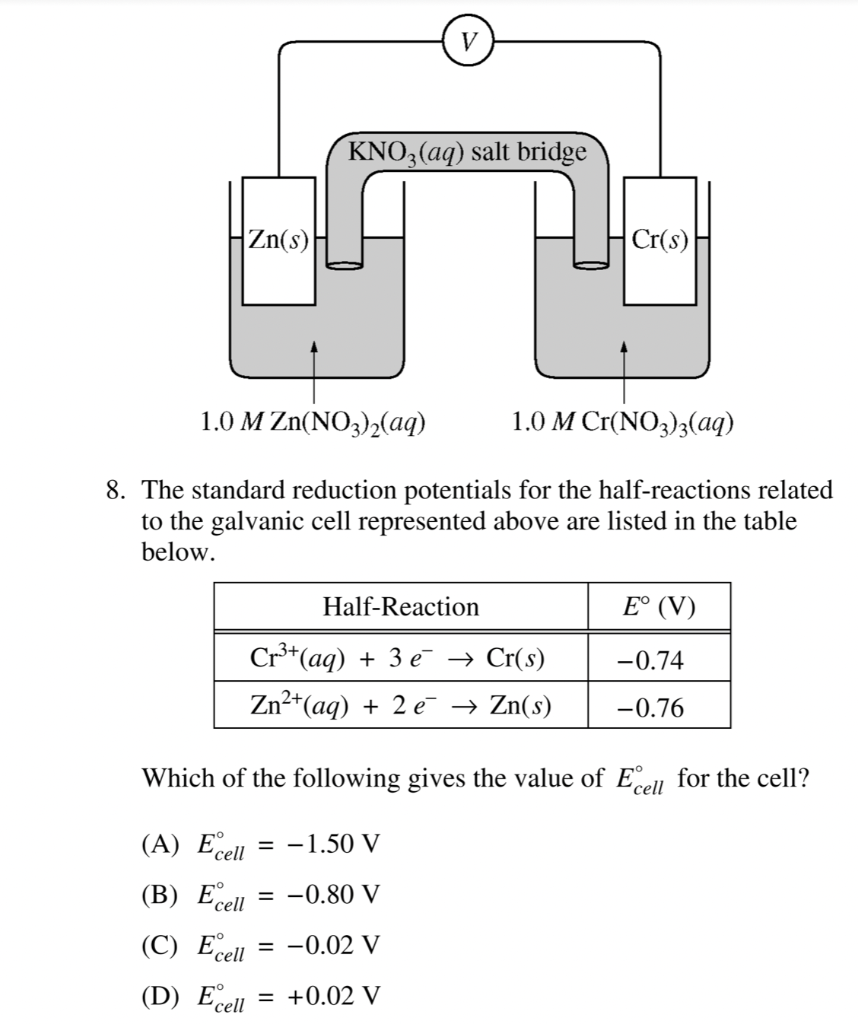
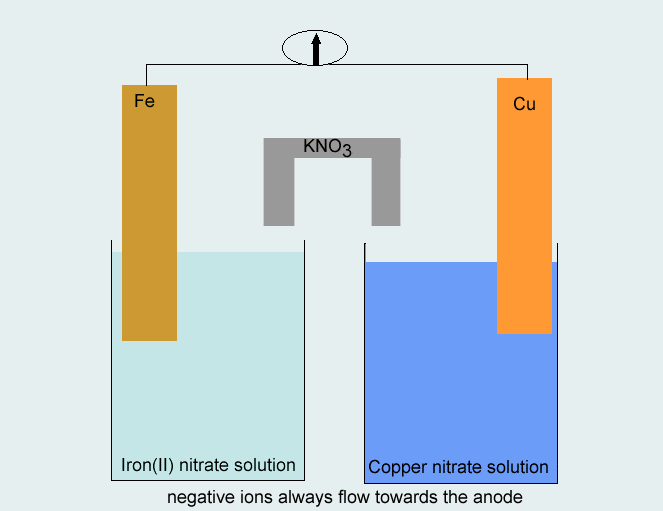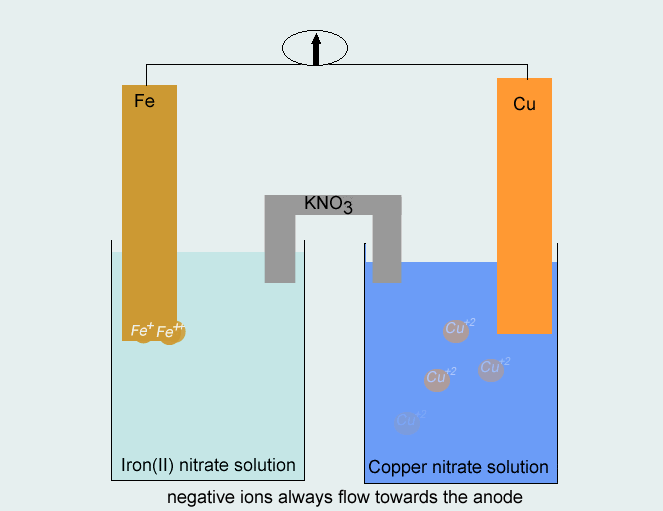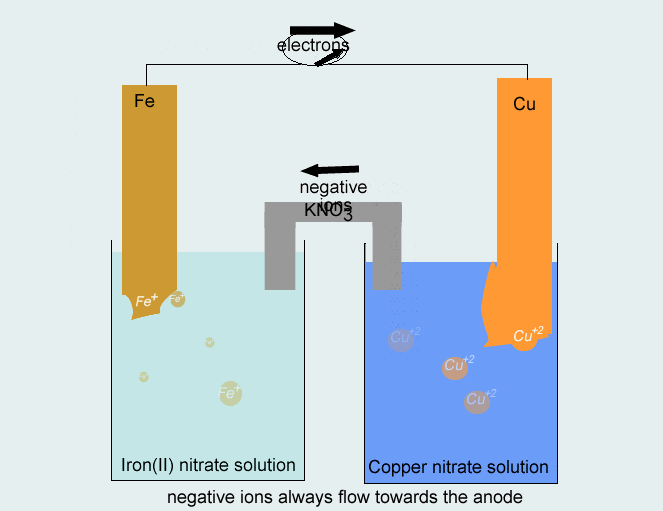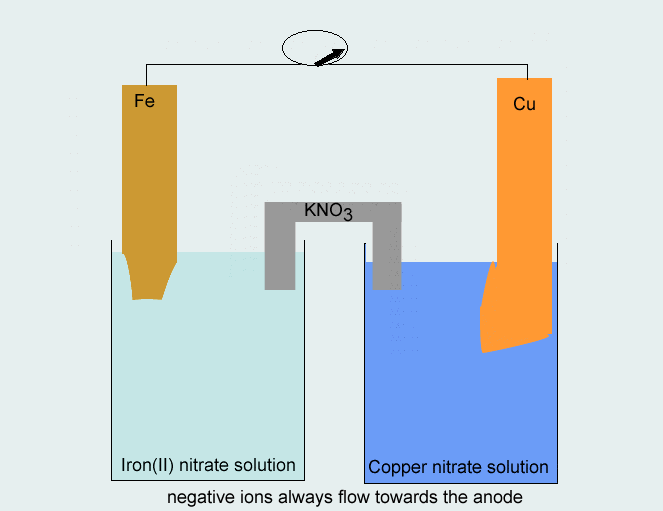
Consider the voltaic cell: 1 M Fe3+ 1 M Cr3+ Fe(s) Cr(s) Salt bridge containing KNO3(aq) | StudySoup

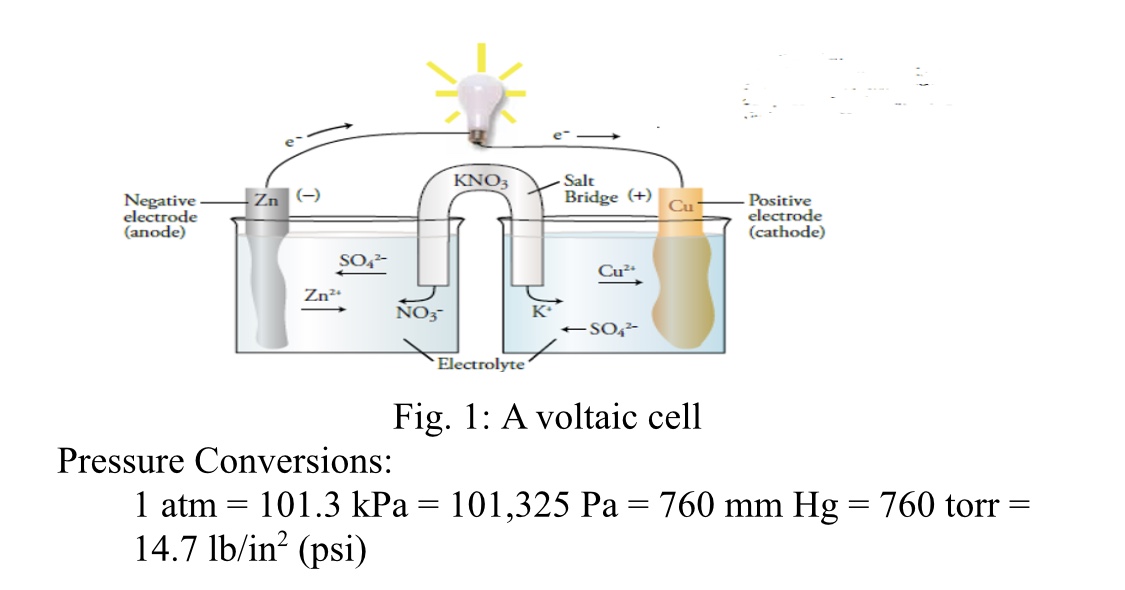
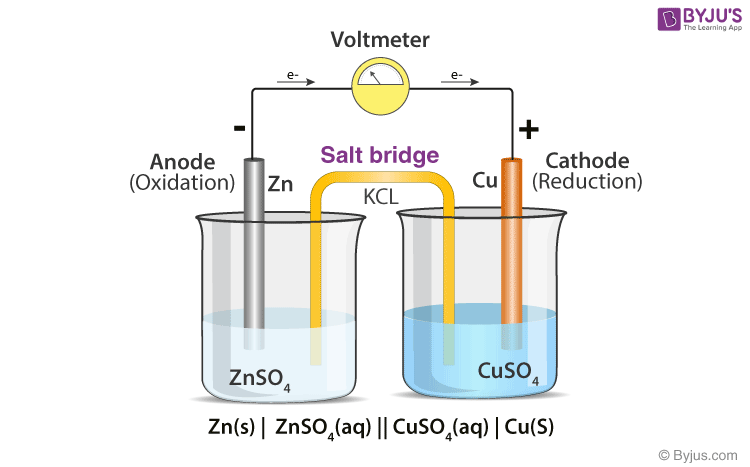
An electrochemical cell is constructed using 1.0 M solutions of Fe(NO3)2 and Sn(NO3)2 and strips of Fe and Sn. A voltmeter is connected to the electrodes and a salt bridge connects the
A student is given a standard galvanic cell, represented above, that has a Cu electrode and a Sn electrode. As current flows thr
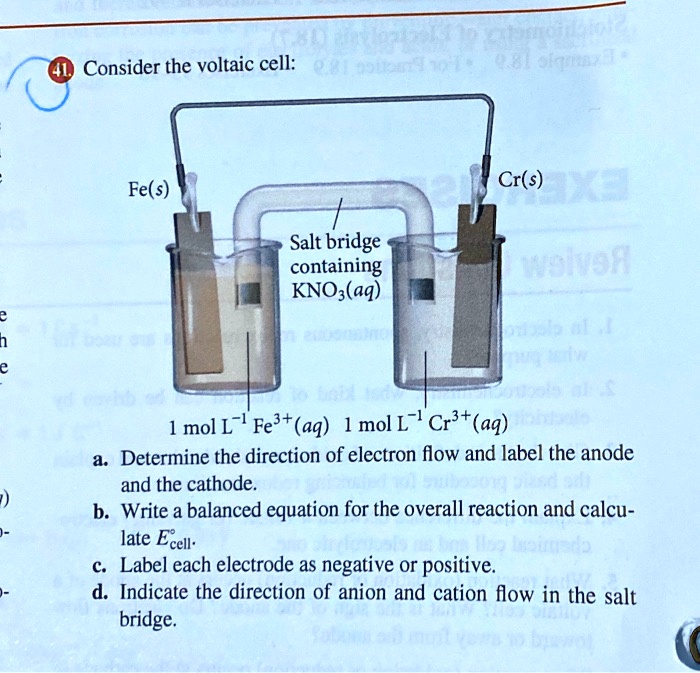
SOLVED: Consider the voltaic cell: Fe(s) Cr(s) Salt bridge containing KNO3(aq) ' mol L-! Fe3+(aq) 1 mol L-! Cr3+(aq) Determine the direction of electron flow and label the anode and the cathode.
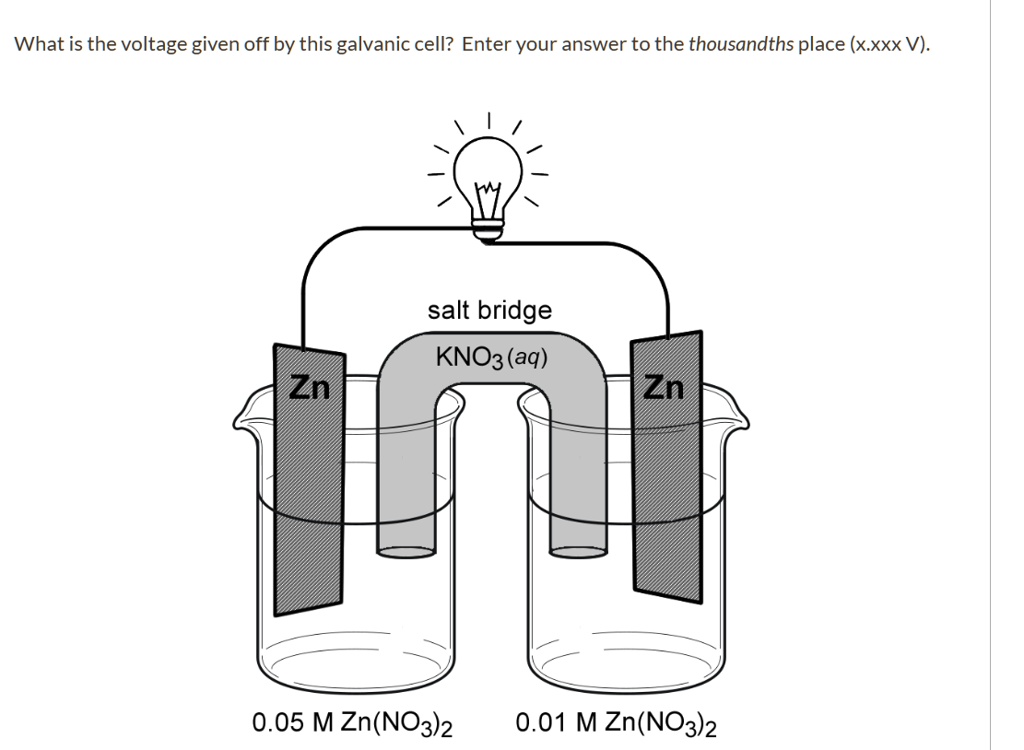

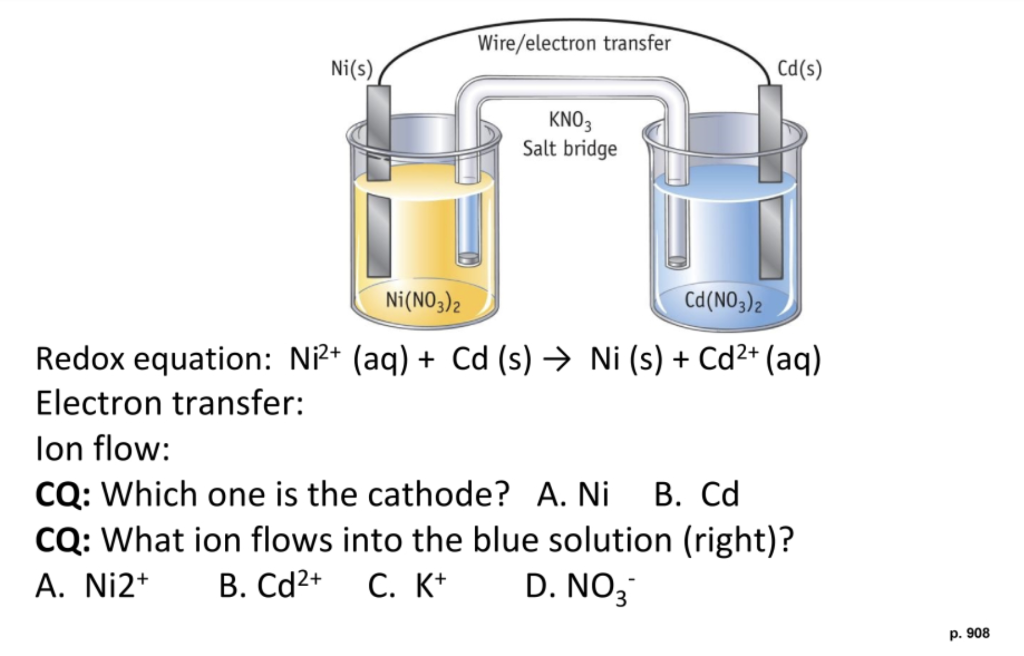

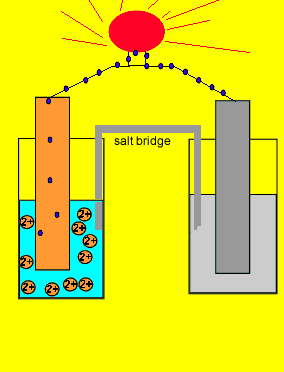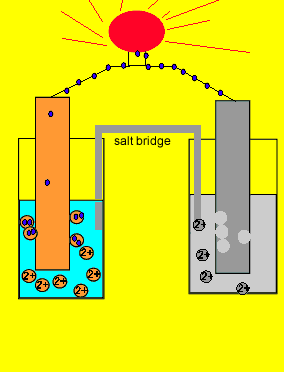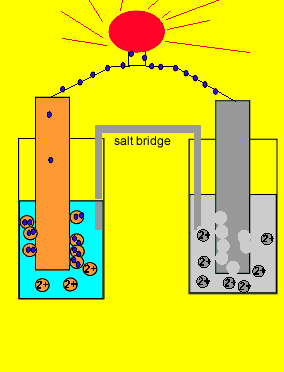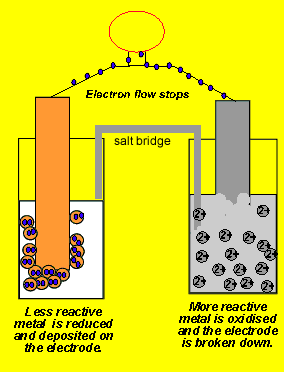

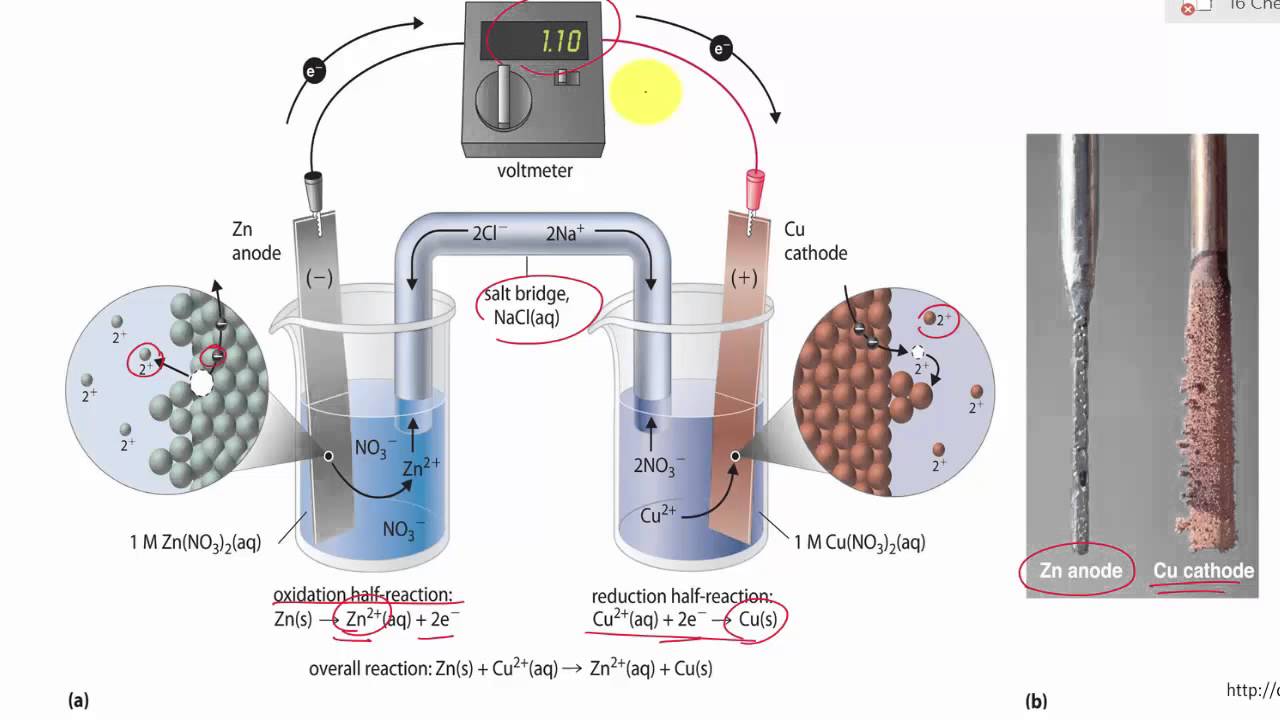
![Solved Voltmeter Wire 0.460 Salt Bridge [KNO3(aq)] NO3 K+ e- | Chegg.com Solved Voltmeter Wire 0.460 Salt Bridge [KNO3(aq)] NO3 K+ e- | Chegg.com](https://media.cheggcdn.com/media/80b/80bf4639-9e4d-4fd2-9391-8ac54bd7d867/php7d36Tc)