Data Integrity Focus, Part VI: Who Is Looking Over Your Shoulder? Quality Oversight for Data Integrity
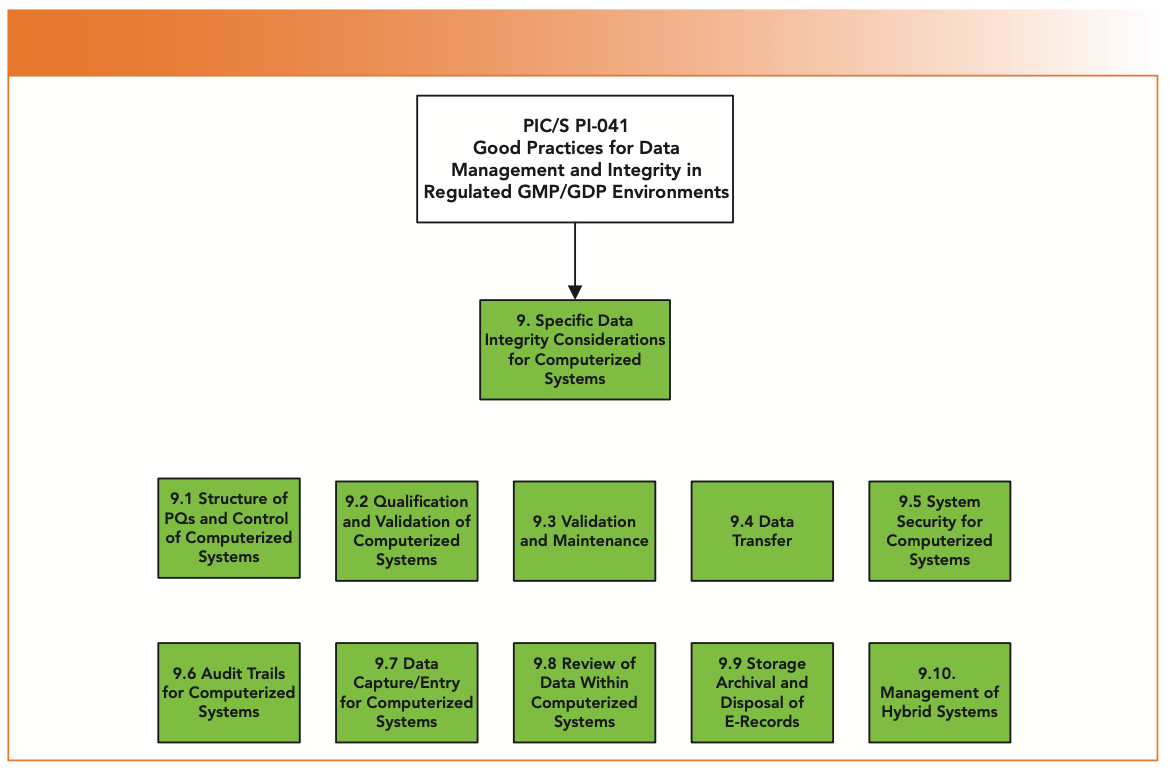
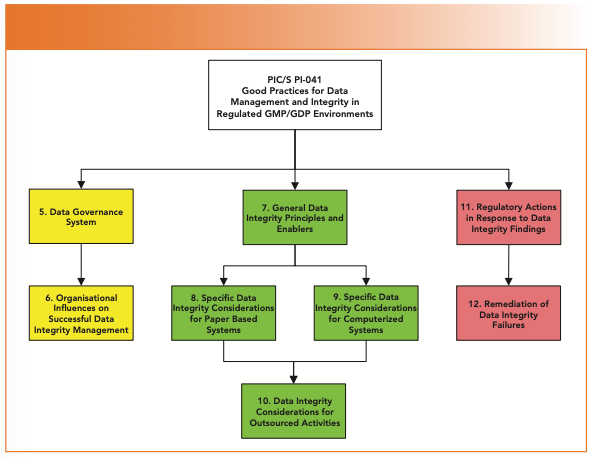
Are You Ready for the Latest Data Integrity Guidance? Part 1: Scope, Data Governance, and Paper Records

Are You Ready for the Latest Data Integrity Guidance? Part 1: Scope, Data Governance, and Paper Records
Are You Ready for the Latest Data Integrity Guidance? Part 1: Scope, Data Governance, and Paper Records

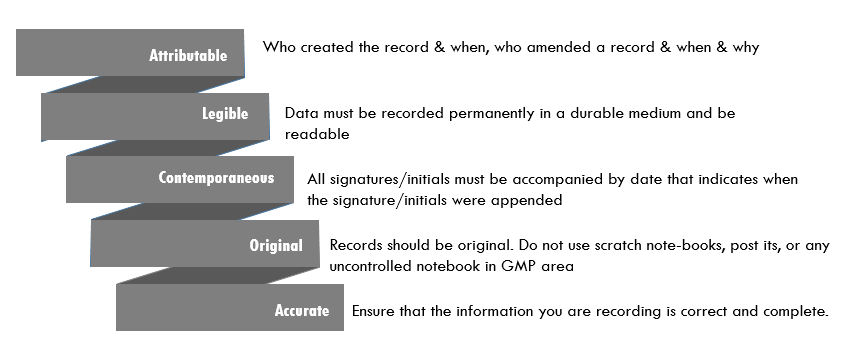
Principles of Data Integrity - Pharmaceutical Guidelines | PDF | Accuracy And Precision | Information Technology
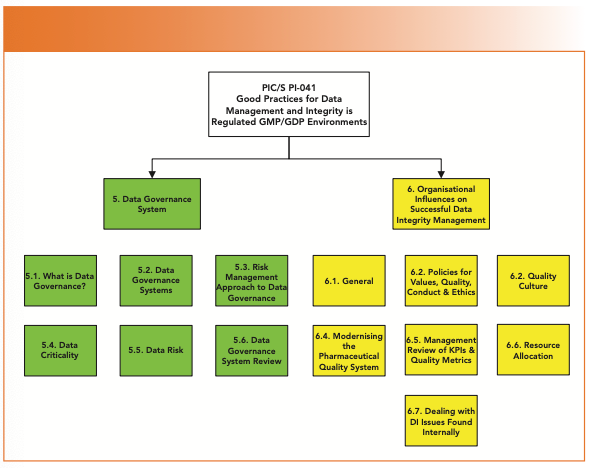
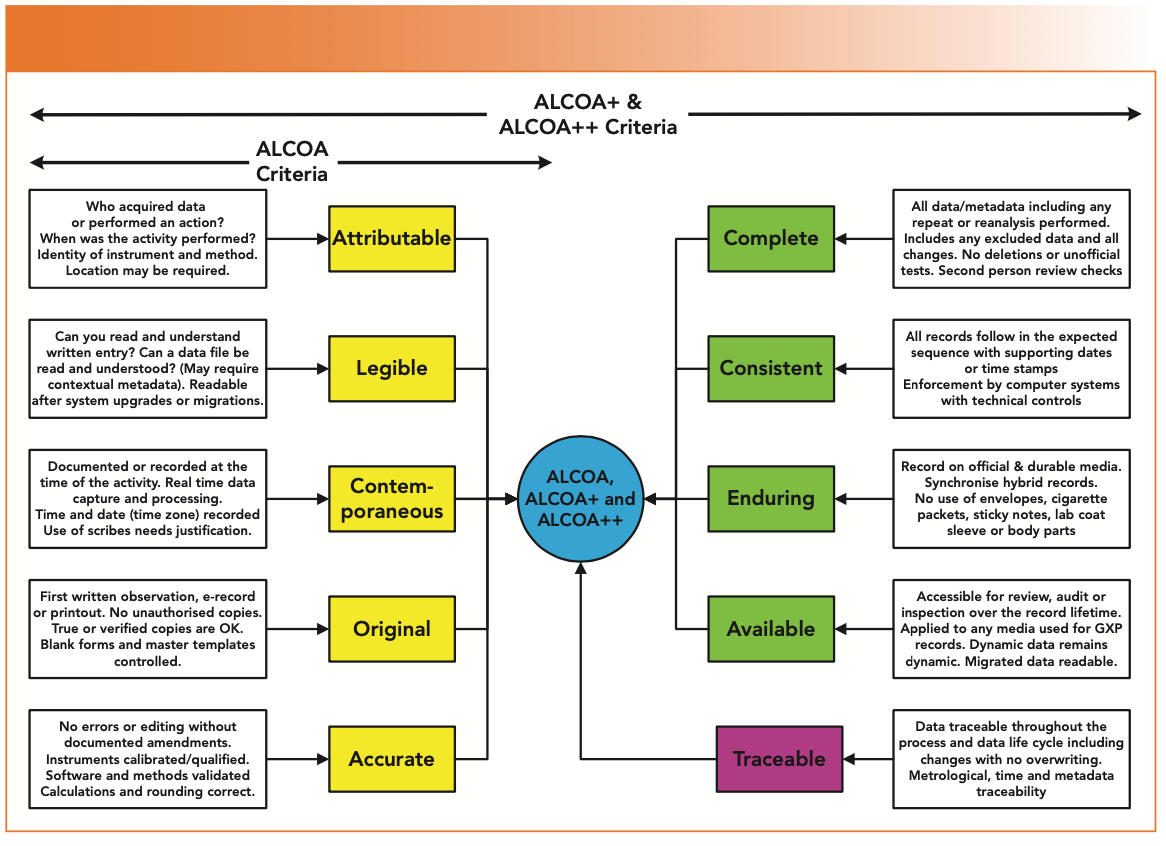
Are You Ready for the Latest Data Integrity Guidance? Part 1: Scope, Data Governance, and Paper Records
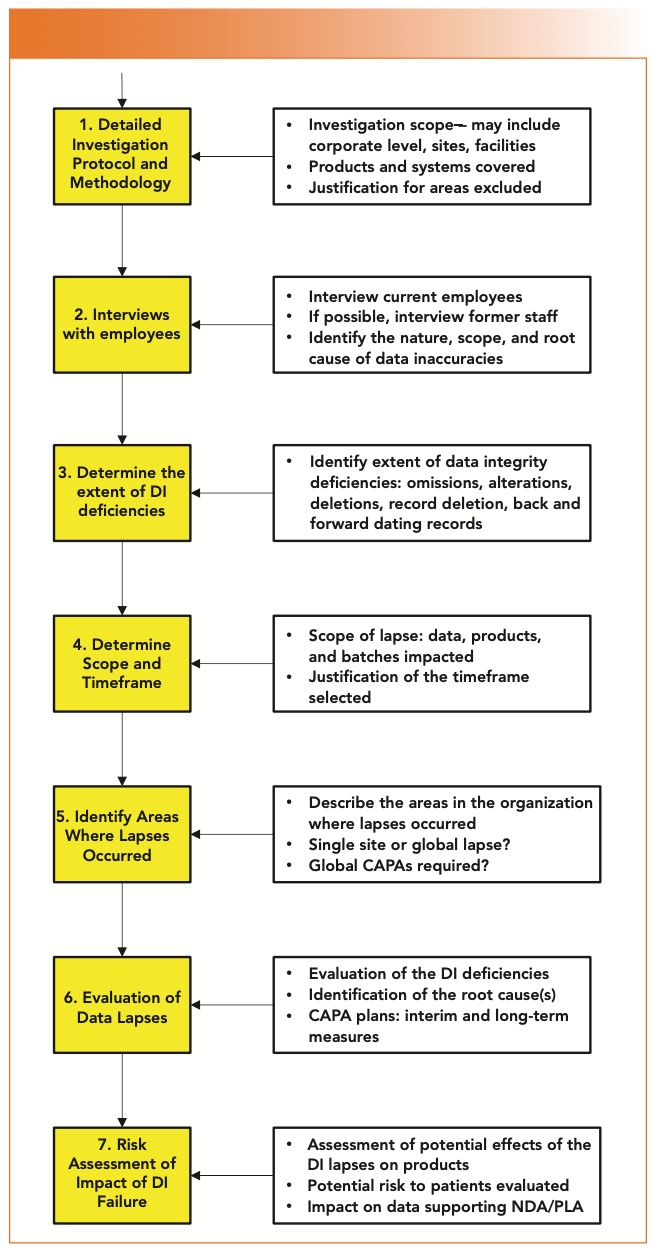
Are You Ready for the Latest Data Integrity Guidance? Part 1: Scope, Data Governance, and Paper Records

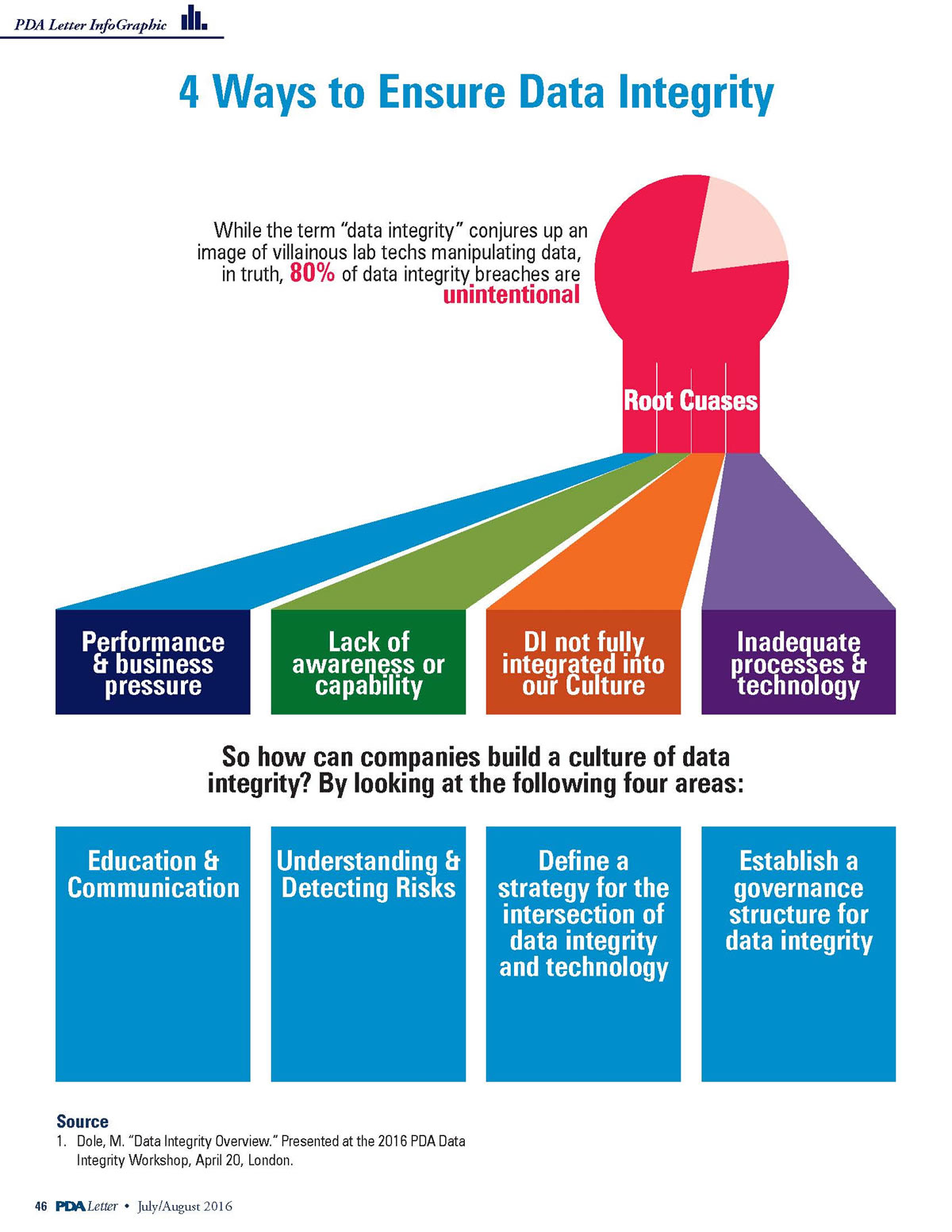
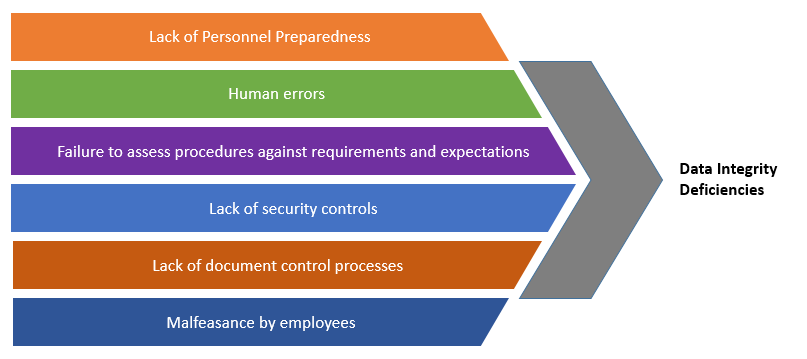
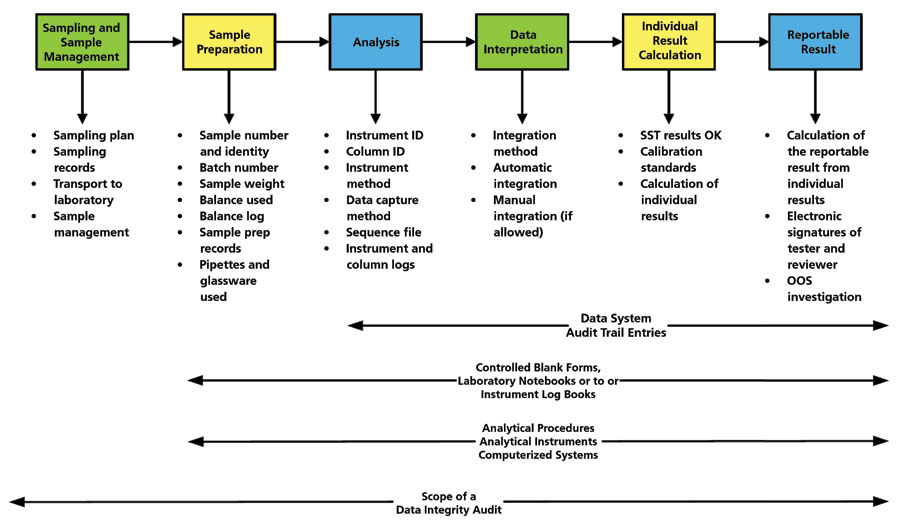
Pharmaceutical Data Integrity: issues, challenges and proposed solutions for manufacturers and inspectors - GaBI Journal
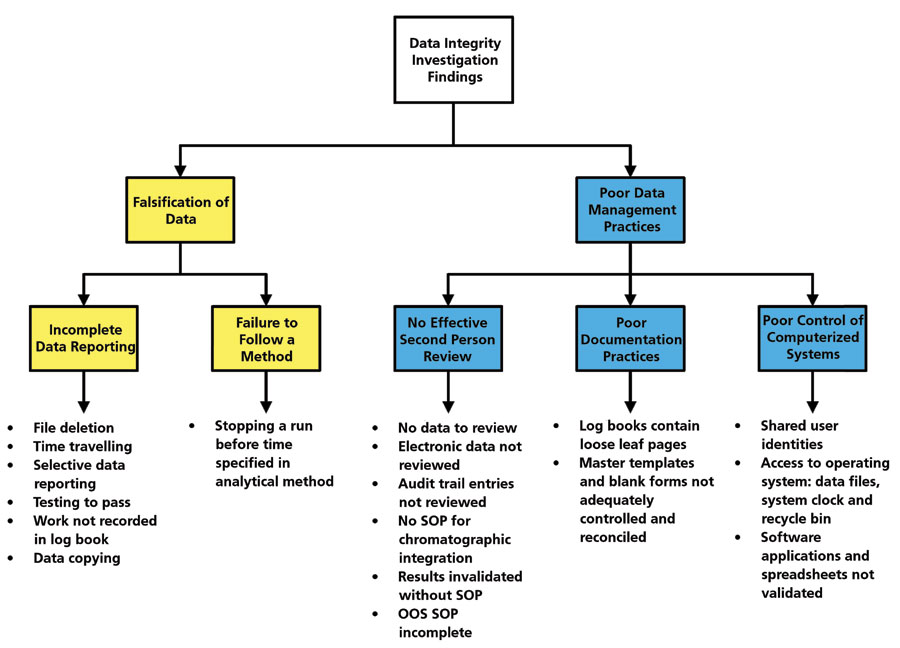
Data Integrity Focus, Part VI: Who Is Looking Over Your Shoulder? Quality Oversight for Data Integrity

Are You Ready for the Latest Data Integrity Guidance? Part 1: Scope, Data Governance, and Paper Records