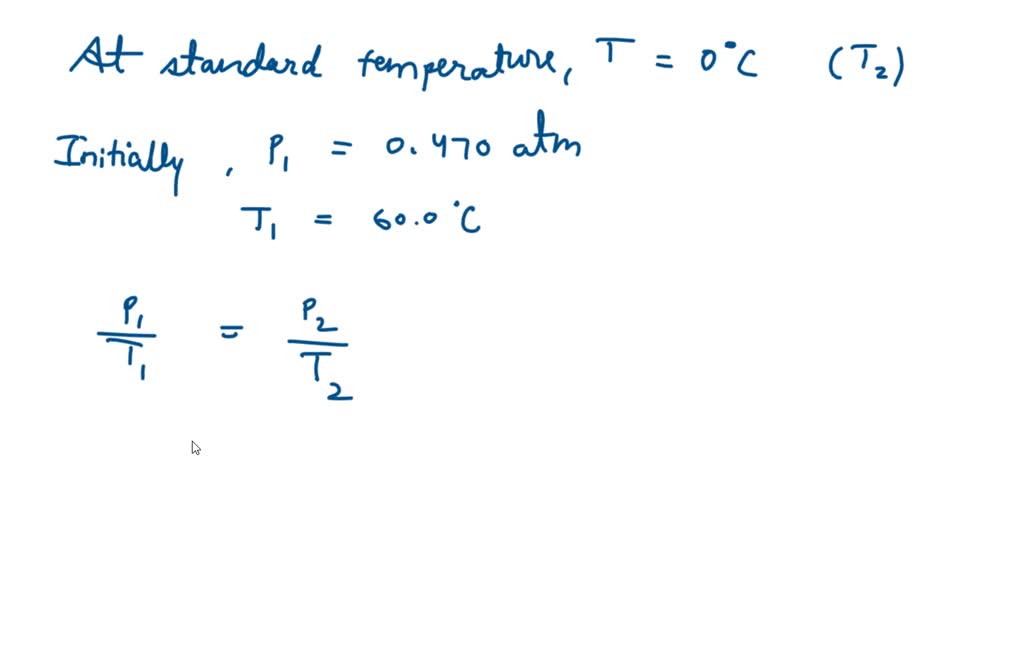Gas Pressure Unit Conversions - torr to atm, psi to atm, atm to mm Hg, kpa to mm Hg, psi to torr - YouTube

Gas Laws. A. Characteristics of Gases Gases expand to fill any container. –random motion, no attraction Gases are fluids (like liquids). –no attraction. - ppt download
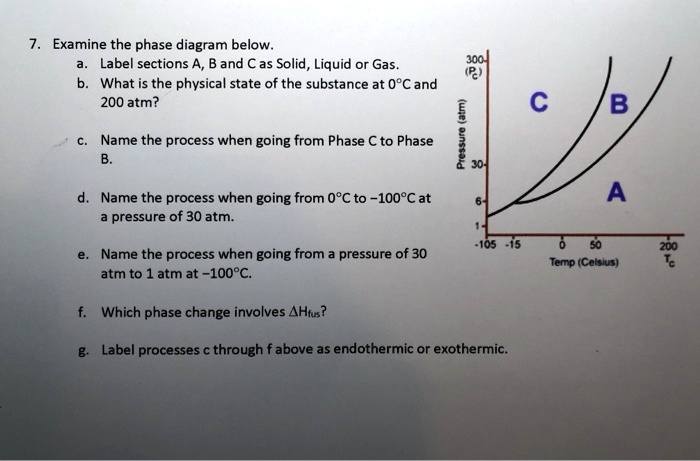
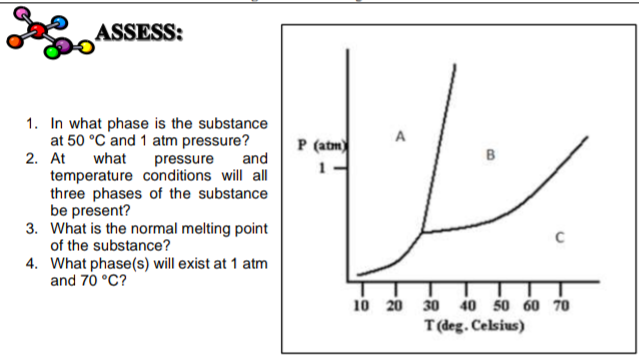
SOLVED: Examine the phase diagram below Label sections A, B and C as Solid, Liquid or Gas. 300 What is the physical state of the substance at 0"C and 200 atm? Name
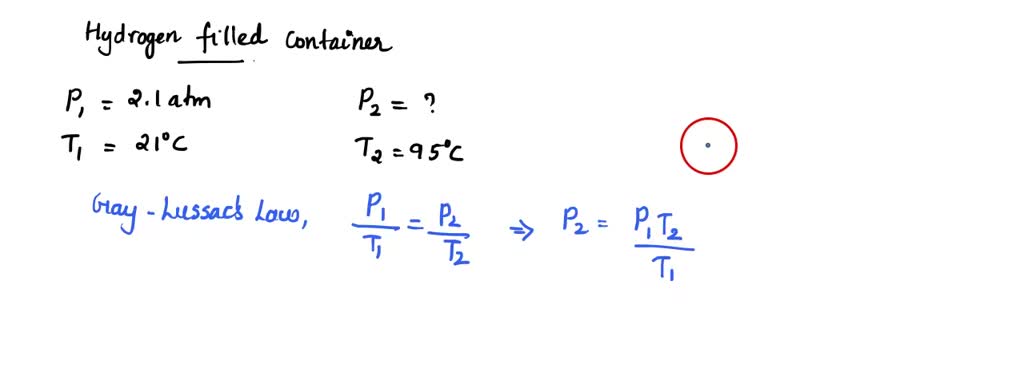
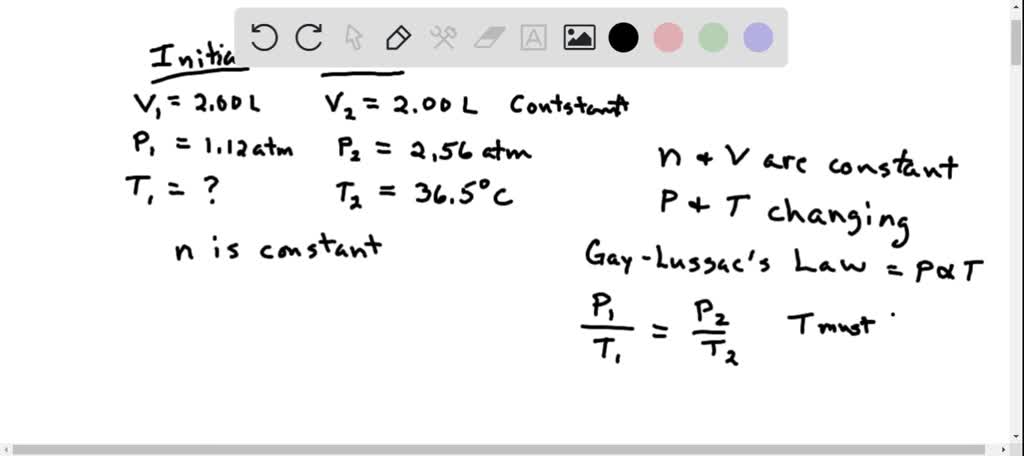
SOLVED: Part A The pressure inside hydrogen-filled container was 2.10 atm at 21 C. What would the pressure be if the container was heated to 95 "C ? Express your answer with

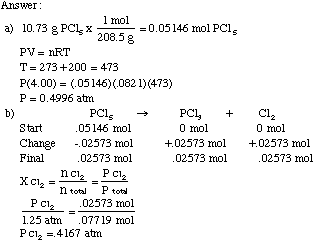
A sample of a gas at 100^(@)C and 0.80 atm pressure has a density of 1.15 g L^(-1). What is the molecular weight of the gas?

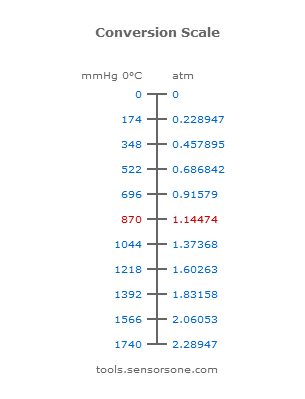
Equivalents for the ways to measures of pressure Atmospheres (atm) Millimeters of mercury (mmHg) – Also known as TORR Kilopascals (kPa) 1 atm = 760 mmHg. - ppt download
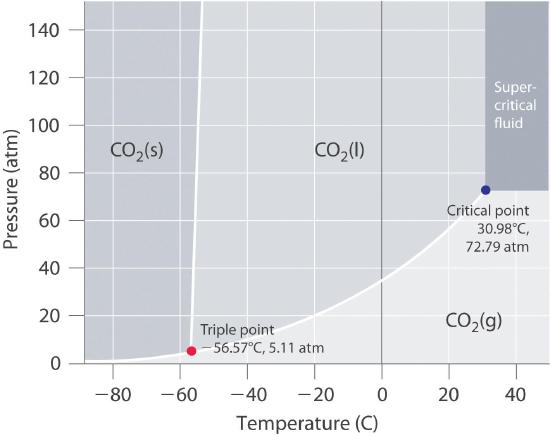
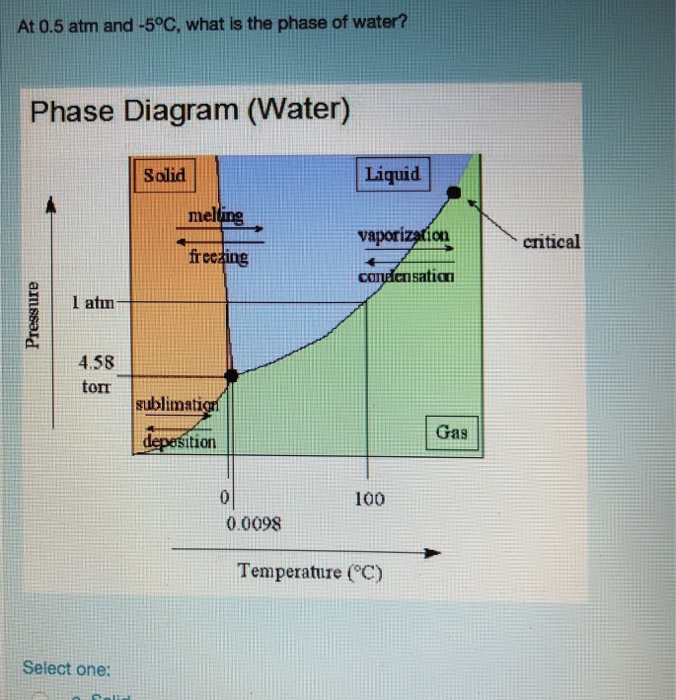
Using the phase diagram for CO_2, what phase is carbon dioxide in at -20 C and 1 atm pressure? | Socratic

SOLVED: How is the pressure in container B related to the pressure in container A? 46. A container is filled with an ideal gas to a pressure of 40.0 atm at 0^∘C.